- Record: found
- Abstract: found
- Article: found
Structure of LP2179, the first representative of Pfam family PF08866, suggests a new fold with a role in amino-acid metabolism
Read this article at
Abstract
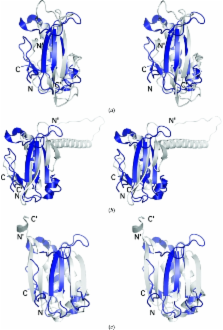
The first structural representative of the PF08866 (DUF1831) protein family reveals a potential new α+β fold and indicates a possible involvement in amino-acid metabolism.
Abstract
The structure of LP2179, a member of the PF08866 (DUF1831) family, suggests a novel α+β fold comprising two β-sheets packed against a single helix. A remote structural similarity to two other uncharacterized protein families specific to the Bacillus genus (PF08868 and PF08968), as well as to prokaryotic S-adenosylmethionine decarboxylases, is consistent with a role in amino-acid metabolism. Genomic neighborhood analysis of LP2179 supports this functional assignment, which might also then be extended to PF08868 and PF08968.