- Record: found
- Abstract: found
- Article: not found
Effects of zoledronic acid versus clodronic acid on skeletal morbidity in patients with newly diagnosed multiple myeloma (MRC Myeloma IX): secondary outcomes from a randomised controlled trial
Read this article at
Summary
Background
Bisphosphonates are the standard of care for reducing the risk of skeletal-related events in patients with bone lesions from multiple myeloma. The MRC Myeloma IX study was designed to compare the effects of zoledronic acid versus clodronic acid in newly diagnosed patients with multiple myeloma. Here, we report the secondary outcomes relating to skeletal events.
Methods
Patients (≥18 years) with newly diagnosed multiple myeloma were enrolled from 120 centres in the UK and received intensive or non-intensive antimyeloma treatment. A computer-generated randomisation sequence was used to allocate patients in a 1:1 ratio, through an automated telephone service to intravenous zoledronic acid (4 mg every 21–28 days) or oral clodronic acid (1600 mg/day), and the drugs were continued at least until disease progression. No investigators, staff, or patients were masked to treatment allocation. The primary endpoints—overall survival, progression-free survival, and overall response rate—and adverse events have been reported previously. We assessed between-group differences with Cox proportional hazards models for time to first skeletal-related event and incidence of skeletal-related events. These were defined as fractures, spinal cord compression, radiation or surgery to bone, and new osteolytic lesions. Data were analysed until disease progression. Analyses were by intention to treat. This trial is registered, number ISRCTN68454111.
Findings
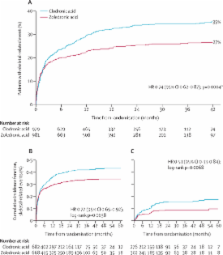
1960 patients were randomly assigned and analysed—981 in the zoledronic acid group and 979 in the clodronic acid group. This trial is fully enrolled, and follow-up continues. At a median follow-up of 3·7 years (IQR 2·9–4·7), patients in the zoledronic acid group had a lower incidence of skeletal-related events than did those in the clodronic acid group (265 [27%] vs 346 [35%], respectively; hazard ratio 0·74, 95% CI 0·62–0·87; p=0·0004). Zoledronic acid was also associated with a lower risk of any skeletal-related event in the subsets of patients with (233 [35%] of 668 vs 292 [43%] of 682 with clodronic acid; 0·77, 0·65–0·92; p=0·0038) and without bone lesions at baseline (29 [10%] of 302 vs 48 [17%] of 276 with clodronic acid; 0·53, 0·33–0·84; p=0·0068). Fewer patients in the zoledronic acid group had vertebral fractures than did those in the clodronic acid group (50 [5%] in the zoledronic acid group vs 88 [9%] in the clodronic acid group; p=0·0008), other fractures (45 [5%] vs 66 [7%]; p=0·04), and new osteolytic lesions (46 [5%] vs 95 [10%]; p<0·0001).
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial
- Record: found
- Abstract: found
- Article: not found
Reduction of osteonecrosis of the jaw (ONJ) after implementation of preventive measures in patients with multiple myeloma treated with zoledronic acid.
- Record: found
- Abstract: found
- Article: not found
