- Record: found
- Abstract: found
- Article: not found
First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial
Read this article at
Summary
Background
Bisphosphonates reduce the risk of skeletal events in patients with malignant bone disease, and zoledronic acid has shown potential anticancer effects in preclinical and clinical studies. We aimed to establish whether bisphosphonates can affect clinical outcomes in patients with multiple myeloma.
Methods
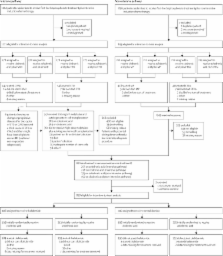
Patients of age 18 years or older with newly diagnosed multiple myeloma were enrolled from 120 centres in the UK. Computer-generated randomisation sequence was used to allocate patients equally, via an automated telephone service, to receive 4 mg zoledronic acid as an infusion every 3–4 weeks or 1600 mg oral clodronic acid daily. Patients also received intensive or non-intensive induction chemotherapy. No investigators, staff, or patients were masked to treatment allocation, and bisphosphonate and maintenance therapy continued at least until disease progression. The primary endpoints were overall survival, progression-free survival, and overall response rate. We assessed between-group differences with Cox proportional hazards models for progression-free survival and overall survival, and with logistic regression models for overall response rate. Analysis was by intention to treat. This trial is registered, number ISRCTN68454111.
Findings
1970 patients were enrolled between May, 2003, and November, 2007, of whom 1960 were eligible for intention-to-treat analysis: 981 in the zoledronic acid group (555 on intensive chemotherapy, 426 on non-intensive chemotherapy); and 979 on clodronic acid (556 on intensive chemotherapy, 423 on non-intensive chemotherapy). The treatment cutoff was Oct 5, 2009, with patients receiving bisphosphonates for a median of 350 days (IQR 137–632) before disease progression, with a median of 3·7 years' follow-up (IQR 2·9–4·7). Zoledronic acid reduced mortality by 16% (95% CI 4–26) versus clodronic acid (hazard ratio [HR] 0·84, 95% CI 0·74–0·96; p=0·0118), and extended median overall survival by 5·5 months (50·0 months, IQR 21·0 to not reached vs 44·5 months, IQR 16·5 to not reached; p=0·04). Zoledronic acid also significantly improved progression-free survival by 12% (95% CI 2–20) versus clodronic acid (HR 0·88, 95% CI 0·80–0·98; p=0·0179), and increased median progression-free survival by 2·0 months (19·5 months, IQR 9·0–38·0 vs 17·5 months, IQR 8·5–34·0; p=0·07). Rates of complete, very good partial, or partial response did not differ significantly between the zoledronic acid and clodronic acid groups for patients receiving intensive induction chemotherapy (432 patients [78%] vs 422 [76%]; p=0·43) or non-intensive induction chemotherapy (215 [50%] vs 195 [46%]; p=0·18). Both bisphosphonates were generally well tolerated, with similar occurrence of acute renal failure and treatment-emergent serious adverse events, but zoledronic acid was associated with higher rates of confirmed osteonecrosis of the jaw (35 [4%]) than was clodronic acid (3 [<1%]).
Interpretation
Consistent with the potential anticancer activity of zoledronic acid, overall survival improved independently of prevention of skeletal-related events, showing that zoledronic acid has treatment benefits beyond bone health. These findings support immediate treatment with zoledronic acid in patients with newly diagnosed multiple myeloma, not only for prevention of skeletal-related events, but also for potential antimyeloma benefits.
Related collections
Most cited references25
- Record: found
- Abstract: found
- Article: not found
Endocrine therapy plus zoledronic acid in premenopausal breast cancer.
- Record: found
- Abstract: found
- Article: not found
Maintenance therapy with thalidomide improves survival in patients with multiple myeloma.
- Record: found
- Abstract: found
- Article: not found
