- Record: found
- Abstract: found
- Article: found
An Updated Review of SARS-CoV-2 Vaccines and the Importance of Effective Vaccination Programs in Pandemic Times
Read this article at
Abstract
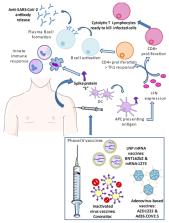
Since the worldwide COVID-19 pandemic was declared a year ago, the search for vaccines has become the top priority in order to restore normalcy after 2.5 million deaths worldwide, overloaded sanitary systems, and a huge economic burden. Vaccine development has represented a step towards the desired herd immunity in a short period of time, owing to a high level of investment, the focus of researchers, and the urge for the authorization of the faster administration of vaccines. Nevertheless, this objective may only be achieved by pursuing effective strategies and policies in various countries worldwide. In the present review, some aspects involved in accomplishing a successful vaccination program are addressed, in addition to the importance of vaccination in a pandemic in the face of unwillingness, conspiracy theories, or a lack of information among the public. Moreover, we provide some updated points related to the landscape of the clinical development of vaccine candidates, specifically, the top five vaccines that are already being assessed in Phase IV clinical trials (BNT162b2, mRNA-1273, AZD1222, Ad26.COV2.S, and CoronaVac).
Related collections
Most cited references165
- Record: found
- Abstract: found
- Article: not found
SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor
- Record: found
- Abstract: found
- Article: not found
Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine
- Record: found
- Abstract: found
- Article: not found