INTRODUCTION
Polytetrafluoroethylene (PTFE), commonly known as Teflon®, is valued for its low coefficient of friction and high inertness. It is used in various technical applications like nonstick coatings. Its elastic modulus describes how the material stretches under stress and is an important characteristic property for engineering purposes. It varies over several orders of magnitude depending on the crystallinity of the material. Characterization of the maximum elastic modulus of PTFE – achievable when the stress acts along the axis of parallel PTFE strands – has received repeated attention over the last decades [1–7]. It was recently estimated to be [5, 7], two orders of magnitude higher than in amorphous PTFE. In this work, we extend previous efforts to determine the highest achievable elastic modulus of polyethylene [8, 9] to PTFE by investigating longitudinal acoustic modes (LAMs) of isolated perfluoro-n-alkanes which are rectified by collisional cooling in supersonic jet expansions. This technique allows us to determine the elastic modulus for cold molecules free of interactions with a molecular environment. The result is well-suited as a benchmark for quantum chemical predictions and sets an upper bound for material studies.
Crystalline PTFE assumes a helical structure [10] which is also confirmed for small perfluoro-n-alkanes in the gas phase [11]. In our jet experiment, we find that the chain molecules exclusively occupy a helical all-anti conformation (vide infra), which is shown in Figure 1 for perfluorotetradecane (n = 14). The LAMs of such fully extended chain molecules have a macroscopic equivalent. For a macroscopic rod of length l, made from a homogeneous material with the elastic modulus E, and density ρ, one finds the following formula for its LAM wavenumbers [12]:
Here, m is the number of nodes of the longitudinal vibration and c0 is the speed of light in vacuum. The vibrational displacement of the LAM-1 is shown in Figure 1 for n = 14. In this work, we present and interpret LAM-1 wavenumbers up to n = 14.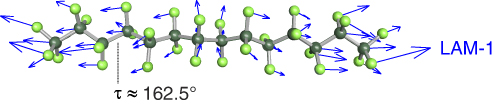
METHODS AND RESULTS
Supersonic expansions of perfluoro-n-alkanes diluted in helium were generated by expanding the gas mixtures from 0.8 bar through a slit nozzle into a vacuum chamber kept at 2 mbar. Gas mixtures were prepared by pre-mixing perfluoro-n-alkanes with helium in a vacuum line (n = 6−9) or by bubbling helium through a coolable glass saturator (n = 10−13, temperature ϑ = −7 to 25°C) or through a heatable brass saturator (n = 14, ϑ = 55°C). The realized mole fractions are in the 0.1% range, but vary somewhat for different chain lengths. This is reflected in a varying signal-to-noise ratio. A 532 nm 18 W continuous-wave laser was focused on the expansion 1 mm downstream the nozzle, perpendicular to the expansion. Raman scattered light was collected at a 90° observation angle. Emission lines from a neon lamp were recorded for wavelength calibration. More details on the Raman jet setup and LAM spectroscopy can be found elsewhere [9]. The resulting Savitzky-Golay filtered (second-order polynomial, 7 pixel) Raman jet spectra are shown in Figure 2 together with harmonic B3LYP-D3/def2-TZVP predictions which were calculated with Turbomole v6.4 [13].

Other than in case of regular n-alkanes [9], conformations with kinks relative to the most stable all-anti conformation do not appear in the spectra. This observation is in line with experimental gauche conformation (CC torsion) energy penalties which are doubled in case of perfluorinated alkanes ( [14, 15] for perfluoro-n-alkanes, [16, 17] for n-alkanes), and with calculated barrier heights, which are smaller for the fluorinated compounds ( [18, 19] for perfluoro-n-alkanes, [20, 21] for n-alkanes), both leading to an enhancement of the ground state population in a jet experiment. Therefore, jet cooling is a perfect alternative to crystal packing in case of perfluoro-n-alkanes.
The observed spectral window reveals the required LAMs with one node between 50 and 200 cm−1. Different kinds of CF2 and CF3 deformation vibrations, partly coupled to C–C stretching vibrations, contribute in the 250–400 cm−1 range and are shown merely for completeness. They will not be analyzed, but the average position of the CF2 scissoring vibration () is used as a wavenumber standard to refine the calibration of the spectra because the wavenumber of this vibration is largely chain length independent (in the B3LYP-D3/def2-TZVP calculations, it scatters around the average value 383.6 cm−1 with a maximum deviation of less than 1 cm−1 for n = 6−14). LAMs with a higher number of nodes are not observed. Notably, some of the LAM-1 bands are split due to harmonic mode mixing with a transverse acoustic mode (TAM). In these cases, the wavenumber of the pure LAM is recovered by determining the intensity-weighted center of the LAM-1 and TAM Raman bands [8] under the assumption that the pure TAM does not carry intensity (I) on its own:
Original and final LAM-1 wavenumbers are summarized in Table 1. The low scattering of the data when plotted appropriately (vide infra) underlines the recalibration and deperturbation success.| Chain length n | 6 | 8 | 9 | 10 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|---|
| Original | 168.3 | 131.4 | 115.7 | 108.3 | 90.2 | 82.9 | 79.8 |
| Original | 141.9 | 116.8 | — | 101.5 | 84.0 | — | — |
| 0.748 | 0.297 | — | 0.257 | 0.215 | — | — | |
| Recalibration | +0.1 | −0.3 | −0.9 | −0.8 | +1.0 | +1.2 | −0.4 |
| Final | 157.1 | 127.7 | 114.8 | 106.1 | 90.5 | 84.1 | 79.4 |
DISCUSSION
Assuming that the chain molecules behave like small homogeneous rods, their LAM-1 wavenumbers can be connected to the elastic modulus E of the regular bulk solid via [8, 12]:
where we use equation (1) and replace the length of the rod l for convenience with the chain length n using the longitudinal segment separation d = 0.130 nm [10] between two –CF2– units (l = nd). In case of long chain molecules, their longitudinal atomic structure is negligible (the homogeneous rod approximation holds) and a plot of LAM-1 wavenumbers versus the inverse chain length yields a straight line [9] whose slope A can be translated to the elastic modulus: LAM-1 wavenumbers of the rather short perfluoroalkanes considered here clearly deviate from linearity when plotted against the inverse chain length due to end-group effects and the curvature of the corresponding frequency branch [22] to which the LAMs belong. We take these deviations into account by slightly rearranging equation (3) and adding an additional term which vanishes for long molecules [8]: Now, the parameter A is the value for in the limit of long chain length. The additional parameters B and C are not given a physical interpretation. The parameters can be derived from a curve fit, but allowing B and C to vary freely raises the uncertainty of A. It is helpful (but not critical) to plug in a quantum chemical estimate for C. This is done by plotting a large set of harmonic HF/STO-3G LAM-1 wavenumbers (n = 6−50, calculated with Gaussian 03 [23]) against (n + C)−2 after deperturbation according to equation (2). For this simulated dataset, C is varied until the quantum chemical predictions fall on a straight line, which is the case for C = 6.5(Figure 3). The restriction to a low computational level is necessary to reach the homogeneous rod limit (high n), which is approached rather late. This is obvious from the alternation for even and odd chain length in case of short molecules, which makes it necessary to split the extrapolation. The validity of this extrapolation approach is strengthened by our studies on n-alkanes where the purely experimental extrapolation of long alkane (n = 16–21) LAM-1 wavenumbers with equation (3) [9] yields virtually the same elastic modulus as the extrapolation of short alkane (n = 5–16) LAM-1 wavenumbers employing low-level quantum chemical predictions and equation (5) [8], 305(5) GPa and 309(8) GPa, respectively.
The experimental extrapolation for perfluoro-n-alkanes with even numbered chain length is shown in Figure 4 (the dataset for odd n being too small) using C = 6.5±1.0 and to establish lower and upper bounds for the y-intercept A, yielding A = 1.21(3)×103 cm−1. With equation (4), the PTFE crystal density [10], ρ = 2.344 g/cm3, and the segment separation [10], d = 0.130 nm, translates to the elastic modulus E = 209(10) GPa.
This Raman jet estimate compares favorably with moduli reported in the literature (Table 2), but the comparison is somewhat hampered by the fact that the literature moduli are reported in part for PTFE in slightly different modifications, and/or use different parameters (density or molecular chain cross-section) to convert molecular properties to the macroscopic modulus. Some literature moduli were derived using densities (or related chain cross-sections) which differ by as much as 10% from the value used here. Our value for E should thus be compared within a larger error margin, including this uncertainty in the density: . This value agrees with most of the values given in Table 2, only the early force field calculation [1] and a stress-strain measurement [3], which might have suffered from a nonuniform distribution of stress over the sample [24], deviate notably.
| Computational | Experimental | |||||||
|---|---|---|---|---|---|---|---|---|
| Reference | [1] | [5] | [6] | [3] | [7] | [2, 4] | [4] | this work |
| E / GPa | 160 | 221 | 247c | 153 | 220 | 222 | 206 | 209(10) |
| Methoda | FF | DFT | DFT | SSb | SSb | LAMb | LAMb | LAM |
FF = force field calculation, DFT = density functional theory calculation, SS = stress-strain measurement, LAM = LAM frequency extrapolation; bsolid state; cplanar backbone.
It is somewhat unsatisfactory to increase the error bars for the more precise spectroscopic data we present here. A way to circumvent the poorly defined density of a single molecular filament (which, in this case, is due to the poorly defined cross-section area S of a molecule) is to translate the spectroscopic value A to the force F which is necessary to stretch a single PTFE rod up to a strain . To do so, we rearrange the definition of the elastic modulus and use equations (3) and (4), and to arrive at:
The cross-section area S cancels, and we are left with the well-defined mass of a 12C19F2 unit [25]. Then, the spectroscopic value A translates to , which reproduces the value we reported [9] for single polyethylene strands F = 5.6(1)×102 pN within the error bars. It should be emphasized that polyethylene prefers a nonhelical all-trans conformation [26]. Apparently, the small twisting of the carbon chain and substitution of hydrogen by fluorine in PTFE does not influence the stiffness of a single chain significantly [3], whereas they contribute to the lower friction by allowing for motion far below the melting transition [27]. The difference in the limiting elastic moduli of PTFE and polyethylene emerges mainly from closer chain packing in case of polyethylene. Other than in case of n-alkanes [8, 9], the modulus derived from condensed phase perfluoroalkane data, extrapolating LAM wavenumbers from solid samples [4], agrees well with our vacuum isolation estimate without corrections for intermolecular interactions [28]. This suggests that such interactions are weaker if hydrogen is swapped with fluorine [29] and is fully in line with the high volatility of perfluoro-n-alkanes.CONCLUSIONS
The longitudinal stiffness of a polymer chain is an important mechanical property which is usually hidden in a macroscopic polymer sample due to conformational flexibility and a lack of regularity. Its extraction from Raman spectra of finite oligomers in the condensed phase may require elaborate corrections for intermolecular interactions [28]. We provide the first interaction-free spectroscopic determination for PTFE from monoconformational, collisionally cooled perfluoro-n-alkanes in supersonic jets. The resulting elastic modulus amounts to two-thirds of the corresponding polyethylene value [8, 9], which is mainly due to the lower chain packing density in PTFE. Single molecular filament characterization in terms of their longitudinal elasticity is now feasible by supersonic jet spectroscopy for fragments with a vapor pressure on the order of at least 10 μbar, if the necessary heating prior to the expansion is tolerated for some hours without decomposition and if the conformational diversity can be controlled by rapid cooling. This adds a gas phase perspective to polymer science which can be exploited for rigorous comparison between theory and experiment.

