- Record: found
- Abstract: found
- Article: found
Multiwalled carbon nanotubes intratracheally instilled into the rat lung induce development of pleural malignant mesothelioma and lung tumors
Read this article at
Abstract
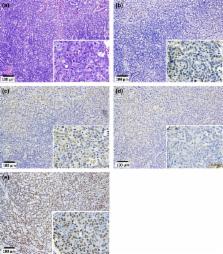
Multiwalled carbon nanotubes (MWCNT) have a fibrous structure and physical properties similar to asbestos and have been shown to induce malignant mesothelioma of the peritoneum after injection into the scrotum or peritoneal cavity in rats and mice. For human cancer risk assessment, however, data after administration of MWCNT via the airway, the exposure route that is most relevant to humans, is required. The present study was undertaken to investigate the carcinogenicity of MWCNT‐N (NIKKISO) after administration to the rat lung. MWCNT‐N was fractionated by passing it through a sieve with a pore size of 25 μm. The average lengths of the MWCNT were 4.2 μm before filtration and 2.6 μm in the flow‐through fraction; the length of the retained MWCNT could not be determined. For the present study, 10‐week‐old F344/Crj male rats were divided into five groups: no treatment, vehicle control, MWCNT‐N before filtration, MWCNT‐N flow‐through and MWCNT‐N retained groups. Administration was by the trans‐tracheal intrapulmonary spraying (TIPS) method. Rats were administered a total of 1 mg/rat during the initial 2 weeks of the experiment and then observed up to 109 weeks. The incidences of malignant mesothelioma and lung tumors (bronchiolo‐alveolar adenomas and carcinomas) were 6/38 and 14/38, respectively, in the three groups administered MWCNT and 0/28 and 0/28, respectively, in the control groups. All malignant mesotheliomas were localized in the pericardial pleural cavity. The sieve fractions did not have a significant effect on tumor incidence. In conclusion, administration of MWCNT to the lung in the rat induces malignant mesothelioma and lung tumors.
Related collections
Most cited references30
- Record: found
- Abstract: found
- Article: not found
Inflammation and cancer: advances and new agents.
- Record: found
- Abstract: found
- Article: not found