- Record: found
- Abstract: found
- Article: found
Quality and Characteristics of 4241 Case Reports of Lactic Acidosis in Metformin Users Reported to a Large Pharmacovigilance Database
Abstract
Objective
Metformin-associated lactic acidosis (MaLA) occurs rarely and is thus difficult to study. We analysed 4241 individual case safety reports of lactic acidosis (LA) that implicated metformin as a suspected drug reported to the pharmacovigilance database of Merck KGaA, Darmstadt, Germany. The primary objective was to review reports for quality and completeness of data to support diagnoses of MaLA. We also explored the correlations between reported biomarkers, and associations between biomarkers and outcomes.
Research Design and Methods
Records were analysed for completeness in supporting diagnoses of LA or metformin-associated LA (MaLA), against commonly used diagnostic criteria. Correlations between indices of exposure to metformin and biomarkers of LA and mortality were investigated.
Results
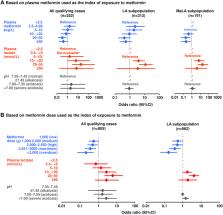
Missing data was common, especially for plasma metformin. Clinical/biomarker evidence supported a diagnosis of LA in only 33% of cases (LA subpopulation) and of MaLA in only 9% (MaLA subpopulation). The metformin plasma level correlated weakly with plasma lactate (positive) and pH (negative). About one-fifth (21.9%) of cases reported a fatal outcome. Metformin exposure (plasma level or dose) was not associated with increased mortality risk (there was a suggestion of decreased risk at higher levels of exposure to metformin). Plasma lactate was the only variable associated with increased risk of mortality. Examination of concomitant risk factors for MaLA identified renal dysfunction (including of iatrogenic origin) as a potential driver of mortality in this population.
Conclusion
Despite the high frequency of missing data, this is the largest analysis of cases of MaLA supported by measurements of circulating metformin, and lactate, and pH, to date. Plasma lactate, and not metformin dose or plasma level, appeared to be the main driver of mortality in the setting of LA or MaLA. Further research with more complete case reports is required.
Most cited references38
- Record: found
- Abstract: not found
- Article: not found
Lactic acidosis.
- Record: found
- Abstract: found
- Article: not found
Metformin in patients with type 2 diabetes and kidney disease: a systematic review.
- Record: found
- Abstract: found
- Article: not found