- Record: found
- Abstract: found
- Article: found
Tissue‐type plasminogen activator (tPA) homozygous Tyr471His mutation associates with thromboembolic disease

Read this article at
Abstract
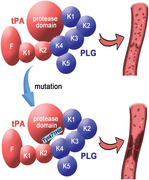
Tissue‐type plasminogen activator (tPA) encoded by PLAT is a major mediator that promotes fibrinolysis and prevents thrombosis. Pathogenetic mutations in PLAT associated with venous thromboembolism have rarely been reported. Here, we report the first case of a homozygous point mutation c.1411T>C (p.Y471H) in PLAT leading to thromboembolic events and conduct related functional studies. The corresponding tPA mutant protein (tPA‐Y471H) and wild‐type tPA (tPA‐WT) were synthesized in vitro, and mutant mice ( PLAT H/H mice) were constructed. The molecular docking and surface plasmon resonance results indicated that the mutation impeded the hydrogen‐bonding interactions between the protease domain of tPA and the kringle 4 domain of plasminogen, and the binding affinity of tPA and plasminogen was significantly reduced with a difference of one order of magnitude. mRNA half‐life assay showed that the half‐life of tPA‐Y471H was shortened. The inferior vena cava thrombosis model showed that the rate of venous thrombosis in PLAT H/H mice was 80% compared with 53% in wild‐type mice. Our data suggested a novel role for the protease domain of tPA in efficient plasminogen activation, and demonstrated that this tPA mutation could reduce the fibrinolysis function of the body and lead to an increased propensity for thrombosis.
Abstract
1. We have identified the first PLAT homozygous mutation (p. Y471H) associated with thromboembolic disease, which is located in the protease domain of tPA and affects the effective activation of plasminogen.
2. Our study might provide a unique target for controlling plasmin generation, and also provides new insights and ideas for the diagnosis and treatment of patients with thrombosis in clinic.
Related collections
Most cited references53
- Record: found
- Abstract: not found
- Article: not found
The HDOCK server for integrated protein–protein docking
- Record: found
- Abstract: found
- Article: not found
PAI-1 in tissue fibrosis.
- Record: found
- Abstract: found
- Article: not found