- Record: found
- Abstract: found
- Article: found
Possible involvement of Interleukin‐17A in the deterioration of prepulse inhibition on acoustic startle response in mice
Read this article at
Abstract
Aim
Proinflammatory cytokines such as interleukin‐6 (IL‐6) and IL‐17A have been implicated in the pathophysiology of schizophrenia which often shows sensorimotor gating abnormalities. This study aimed to examine whether a proinflammatory cytokine, IL‐17A, induces impairment in sensorimotor gating in mice. We also examined whether IL‐17A administration affects GSK3α/β protein level or phosphorylation in the striatum.
Methods
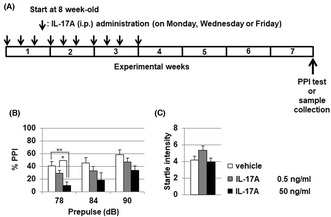
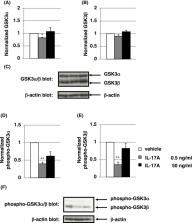
Recombinant mouse IL‐17A (low‐dose: 0.5 ng/mL and high‐dose: 50 ng/mL with 10 μL/g mouse body weight, respectively) or vehicle was intraperitoneally administered into C57BL/6 male mice 10 times in 3 weeks (sub‐chronic administration). Prepulse inhibition test using acoustic startle stimulus was conducted 4 weeks after the final IL‐17A administration. We evaluated the effect of IL‐17A administration on protein level or phosphorylation of GSK3α/β in the striatum by using Western blot analysis.
Results
Administration of IL‐17A induced significant PPI deterioration. Low‐dose of IL‐17A administration significantly decreased both GSK3α (Ser21) and GSK3β (Ser9) phosphorylation in mouse striatum. There was no significant alteration of GSK3α/β protein levels except for GSK3α in low‐dose IL‐17A administration group.
Conclusion
We demonstrated for the first time that sub‐chronic IL‐17A administration induced PPI disruption and that IL‐17A administration resulted in decreased phosphorylation of GSKα/β at the striatum. These results suggest that IL‐17A could be a target molecule in the prevention and treatment of sensorimotor gating abnormalities observed in schizophrenia.
Abstract
We demonstrated for the first time that sub‐chronic IL‐17A administration induced PPI disruption. IL‐17A administration resulted in decreased phosphorylation of GSKa/ß at the striatum. These results suggest that IL‐17A could be a target molecule in the prevention and treatment of sensorimotor gating abnormalities observed in schizophrenia.
Related collections
Most cited references60
- Record: found
- Abstract: found
- Article: not found
Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells.
- Record: found
- Abstract: found
- Article: not found
A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression
- Record: found
- Abstract: found
- Article: not found