- Record: found
- Abstract: found
- Article: found
Application of preoperative ultrasound features combined with clinical factors in predicting HER2-positive subtype (non-luminal) breast cancer
Read this article at
Abstract
Background
Human epidermal growth factor receptor2+ subtype breast cancer has a high degree of malignancy and a poor prognosis. The aim of this study is to develop a prediction model for the human epidermal growth factor receptor2+ subtype (non-luminal) of breast cancer based on the clinical and ultrasound features related with estrogen receptor, progesterone receptor, and human epidermal growth factor receptor2.
Methods
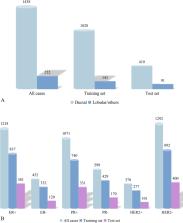
We collected clinical data and reviewed preoperative ultrasound images of enrolled breast cancers from September 2017 to August 2020. We divided the data into in three groups as follows. Group I: estrogen receptor ± , Group II: progesterone receptor ± and Group III: human epidermal growth factor receptor2 ± . Univariate and multivariate logistic regression analyses were used to analyze the clinical and ultrasound features related with biomarkers among these groups. A model to predict human epidermal growth factor receptor2+ subtype was then developed based on the results of multivariate regression analyses, and the efficacy was evaluated using the area under receiver operating characteristic curve, accuracy, sensitivity, specificity.
Results
The human epidermal growth factor receptor2+ subtype accounted for 138 cases (11.8%) in the training set and 51 cases (10.1%) in the test set. In the multivariate regression analysis, age ≤ 50 years was an independent predictor of progesterone receptor + (p = 0.007), and posterior enhancement was a negative predictor of progesterone receptor + (p = 0.013) in Group II; palpable axillary lymph node, round, irregular shape and calcifications were independent predictors of the positivity for human epidermal growth factor receptor-2 in Group III (p = 0.001, p = 0.007, p = 0.010, p < 0.001, respectively). In Group I, shape was the only factor related to estrogen receptor status in the univariate analysis (p < 0.05). The area under receiver operating characteristic curve, accuracy, sensitivity, specificity of the model to predict human epidermal growth factor receptor2+ subtype breast cancer was 0.697, 60.14%, 72.46%, 58.49% and 0.725, 72.06%, 64.71%, 72.89% in the training and test sets, respectively.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: not found
Cancer statistics, 2020

- Record: found
- Abstract: found
- Article: found
Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013
- Record: found
- Abstract: found
- Article: not found