- Record: found
- Abstract: found
- Article: found
Knowledge-based planning for multi-isocenter VMAT total marrow irradiation
Read this article at
Abstract
Purpose
Total marrow irradiation (TMI) involves optimization of extremely large target volumes and requires extensive clinical experience and time for both treatment planning and delivery. Although volumetric modulated arc therapy (VMAT) achieves substantial reduction in treatment delivery time, planning process still presents a challenge due to use of multiple isocenters and multiple overlapping arcs. We developed and evaluated a knowledge-based planning (KBP) model for VMAT-TMI to address these clinical challenges.
Methods
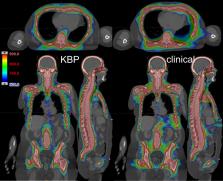
Fifty-one patients previously treated in our clinic were selected for the model training, while 22 patients from another clinic were used as a test set. All plans used a 3-isocenter to cover sub-target volumes of head and neck (HN), chest, and pelvis. Chest plan was performed first and then used as the base dose for both the HN and pelvis plans to reduce hot spots around the field junctions. This resulted in a wide range of dose-volume histograms (DVH). To address this, plans without the base-dose plan were optimized and added to the library to train the model.
Results
KBP achieved our clinical goals (95% of PTV receives 100% of Rx) in a single day, which used to take 4-6 days of effort without KBP. Statistically significant reductions with KBP were observed in the mean dose values to brain, lungs, oral cavity and lenses. KBP substantially improved 105% dose spillage (14.1% ± 2.4% vs 31.8% ± 3.8%), conformity index (1.51 ± 0.06 vs 1.81 ± 0.12) and homogeneity index (1.25 ± 0.02 vs 1.33 ± 0.03).
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Predicting dose-volume histograms for organs-at-risk in IMRT planning.
- Record: found
- Abstract: found
- Article: not found
A planning quality evaluation tool for prostate adaptive IMRT based on machine learning.
- Record: found
- Abstract: found
- Article: not found