- Record: found
- Abstract: found
- Article: not found
Interventions for Menière’s disease: an umbrella systematic review
Abstract
Objectives
To systematically review the efficacy of interventions for Menière’s disease (MD) to report clinical implications of the results and to identify areas for future valuable research.
Methods
In line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Abstracts (PRISMA-A) guideline, a systematic online database search was conducted in which MEDLINE (PubMed), Embase (Ovid) and CENTRAL (Cochrane Library) were searched until May 2021 in order to search for the efficacy of treatment was analysed in a systematic review. Systematic reviews (SRs) on treatments for MD were screened for eligible interventions. From these SRs, we included placebo randomised controlled trials (RCTs). A separate search was conducted to identify RCTs on treatment modalities that were systematically reviewed yet published after the conduction of these SRs. The primary outcome was control of vertigo as defined by the American guideline as published in 1995. The PRISMA-A and the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach was used to appraise and evaluate the certainty of evidence.
Results
We found five SRs from which 19 RCTs were extracted. Five RCTs were added by the separate search resulting in a total of 25 RCTs (n=1248) which evaluated the efficacy of betahistine dihydrochloride, intratympanic injections with gentamicin or steroids, endolymphatic sac surgery and pressure pulse therapy. Evidence on the efficacy of interventions for patients with MD is generally of low certainty. Betahistine (48 mg per day and 144 mg per day) and positive pressure therapy probably do not reduce MD symptoms when compared with placebo. Intratympanic injection with gentamicin or steroids, or treatment with endolymphatic surgery may reduce symptoms in MD when compared with placebo.
Related collections
Most cited references50

- Record: found
- Abstract: found
- Article: found
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews
- Record: found
- Abstract: found
- Article: found
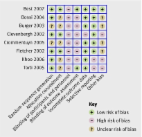
The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials
- Record: found
- Abstract: found
- Article: found