- Record: found
- Abstract: found
- Article: found
Micromechanical modeling of the contact stiffness of an osseointegrated bone–implant interface

Read this article at
Abstract
Background
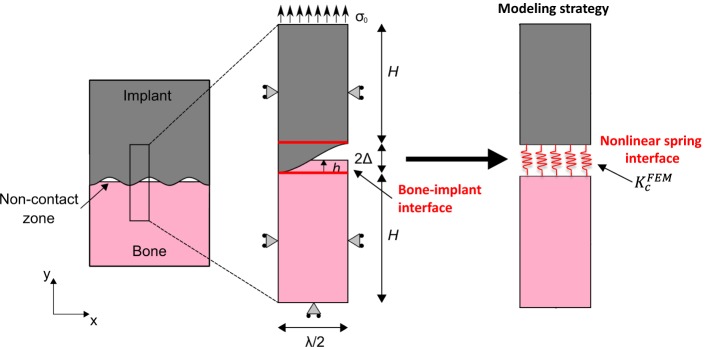
The surgical success of cementless implants is determined by the evolution of the biomechanical properties of the bone–implant interface (BII). One difficulty to model the biomechanical behavior of the BII comes from the implant surface roughness and from the partial contact between bone tissue and the implant. The determination of the constitutive law of the BII would be of interest in the context of implant finite element (FE) modeling to take into account the imperfect characteristics of the BII. The aim of the present study is to determine an effective contact stiffness \documentclass[12pt]{minimal} \usepackage{amsmath} \usepackage{wasysym} \usepackage{amsfonts} \usepackage{amssymb} \usepackage{amsbsy} \usepackage{mathrsfs} \usepackage{upgreek} \setlength{\oddsidemargin}{-69pt} \begin{document}$$\left( {K_{c}^{\text{FEM}} } \right)$$\end{document} of an osseointegrated BII accounting for its micromechanical features such as surface roughness, bone–implant contact ratio (BIC) and periprosthetic bone properties. To do so, a 2D FE model of the BII under normal contact conditions was developed and was used to determine the behavior of \documentclass[12pt]{minimal} \usepackage{amsmath} \usepackage{wasysym} \usepackage{amsfonts} \usepackage{amssymb} \usepackage{amsbsy} \usepackage{mathrsfs} \usepackage{upgreek} \setlength{\oddsidemargin}{-69pt} \begin{document}$$K_{c}^{\text{FEM}}$$\end{document} .
Results
The model is validated by comparison with three analytical schemes based on micromechanical homogenization including two Lekesiz’s models (considering interacting and non-interacting micro-cracks) and a Kachanov’s model. \documentclass[12pt]{minimal} \usepackage{amsmath} \usepackage{wasysym} \usepackage{amsfonts} \usepackage{amssymb} \usepackage{amsbsy} \usepackage{mathrsfs} \usepackage{upgreek} \setlength{\oddsidemargin}{-69pt} \begin{document}$$K_{c}^{\text{FEM}}$$\end{document} is found to be comprised between 10 13 and 10 15 N/m 3 according to the properties of the BII. \documentclass[12pt]{minimal} \usepackage{amsmath} \usepackage{wasysym} \usepackage{amsfonts} \usepackage{amssymb} \usepackage{amsbsy} \usepackage{mathrsfs} \usepackage{upgreek} \setlength{\oddsidemargin}{-69pt} \begin{document}$$K_{c}^{\text{FEM}}$$\end{document} is shown to increase nonlinearly as a function of the BIC and to decrease as a function of the roughness amplitude for high BIC values (above around 20%). Moreover, \documentclass[12pt]{minimal} \usepackage{amsmath} \usepackage{wasysym} \usepackage{amsfonts} \usepackage{amssymb} \usepackage{amsbsy} \usepackage{mathrsfs} \usepackage{upgreek} \setlength{\oddsidemargin}{-69pt} \begin{document}$$K_{c}^{\text{FEM}}$$\end{document} decreases as a function of the roughness wavelength and increases linearly as a function of the Young’s modulus of periprosthetic bone tissue.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: not found
Osteoinduction, osteoconduction and osseointegration.
- Record: found
- Abstract: found
- Article: not found
Observations on the effect of movement on bone ingrowth into porous-surfaced implants.
- Record: found
- Abstract: found
- Article: not found