- Record: found
- Abstract: found
- Article: found
Nucleoporin Nup62 maintains centrosome homeostasis
Read this article at
Abstract
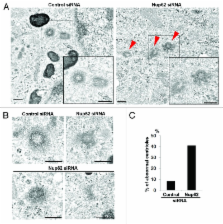
Centrosomes are comprised of 2 orthogonally arranged centrioles surrounded by the pericentriolar material (PCM), which serves as the main microtubule organizing center of the animal cell. More importantly, centrosomes also control spindle polarity and orientation during mitosis. Recently, we and other investigators discovered that several nucleoporins play critical roles during cell division. Here, we show that nucleoporin Nup62 plays a novel role in centrosome integrity. Knockdown of Nup62 induced mitotic arrest in G 2/M phases and mitotic cell death. Depletion of Nup62 using RNA interference results in defective centrosome segregation and centriole maturation during the G 2 phase. Moreover, Nup62 depletion in human cells leads to the appearance of multinucleated cells and induces the formation of multipolar centrosomes, centriole synthesis defects, dramatic spindle orientation defects, and centrosome component rearrangements that impair cell bi-polarity. Our results also point to a potential role of Nup62 in targeting gamma-tubulin and SAS-6 to the centrioles.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
Proteomic characterization of the human centrosome by protein correlation profiling.
- Record: found
- Abstract: found
- Article: not found
Centrioles, centrosomes, and cilia in health and disease.
- Record: found
- Abstract: found
- Article: not found