- Record: found
- Abstract: found
- Article: found
Advanced Parkinson’s or “complex phase” Parkinson’s disease? Re-evaluation is needed
Read this article at
Abstract
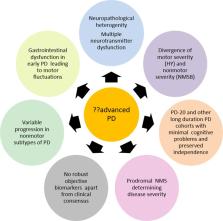
Holistic management of Parkinson’s disease, now recognised as a combined motor and nonmotor disorder, remains a key unmet need. Such management needs relatively accurate definition of the various stages of Parkinson’s from early untreated to late palliative as each stage calls for personalised therapies. Management also needs to have a robust knowledge of the progression pattern and clinical heterogeneity of the presentation of Parkinson’s which may manifest in a motor dominant or nonmotor dominant manner. The “advanced” stages of Parkinson’s disease qualify for advanced treatments such as with continuous infusion or stereotactic surgery yet the concept of “advanced Parkinson’s disease” (APD) remains controversial in spite of growing knowledge of the natural history of the motor syndrome of PD. Advanced PD is currently largely defined on the basis of consensus opinion and thus with several caveats. Nonmotor aspects of PD may also reflect advancing course of the disorder, so far not reflected in usual scale based assessments which are largely focussed on motor symptoms. In this paper, we discuss the problems with current definitions of “advanced” PD and also propose the term “complex phase” Parkinson’s disease as an alternative which takes into account a multimodal symptoms and biomarker based approach in addition to patient preference.
Related collections
Most cited references44
- Record: found
- Abstract: found
- Article: not found
The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years.
- Record: found
- Abstract: found
- Article: not found
The clinical symptoms of Parkinson's disease.
- Record: found
- Abstract: found
- Article: not found