- Record: found
- Abstract: found
- Article: found
Effectiveness of interventions to improve drinking water, sanitation, and handwashing with soap on risk of diarrhoeal disease in children in low-income and middle-income settings: a systematic review and meta-analysis
Read this article at
Summary
Background
Estimates of the effectiveness of water, sanitation, and hygiene (WASH) interventions that provide high levels of service on childhood diarrhoea are scarce. We aimed to provide up-to-date estimates on the burden of disease attributable to WASH and on the effects of different types of WASH interventions on childhood diarrhoea in low-income and middle-income countries (LMICs).
Methods
In this systematic review and meta-analysis, we updated previous reviews following their search strategy by searching MEDLINE, Embase, Scopus, Cochrane Library, and BIOSIS Citation Index for studies of basic WASH interventions and of WASH interventions providing a high level of service, published between Jan 1, 2016, and May 25, 2021. We included randomised and non-randomised controlled trials conducted at household or community level that matched exposure categories of the so-called service ladder approach of the Sustainable Development Goal (SDG) for WASH. Two reviewers independently extracted study-level data and assessed risk of bias using a modified Newcastle-Ottawa Scale and certainty of evidence using a modified Grading of Recommendations, Assessment, Development, and Evaluation approach. We analysed extracted relative risks (RRs) and 95% CIs using random-effects meta-analyses and meta-regression models. This study is registered with PROSPERO, CRD42016043164.
Findings
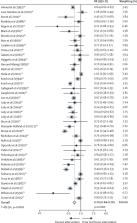
19 837 records were identified from the search, of which 124 studies were included, providing 83 water (62 616 children), 20 sanitation (40 799 children), and 41 hygiene (98 416 children) comparisons. Compared with untreated water from an unimproved source, risk of diarrhoea was reduced by up to 50% with water treated at point of use (POU): filtration (n=23 studies; RR 0·50 [95% CI 0·41–0·60]), solar treatment (n=13; 0·63 [0·50–0·80]), and chlorination (n=25; 0·66 [0·56–0·77]). Compared with an unimproved source, provision of an improved drinking water supply on premises with higher water quality reduced diarrhoea risk by 52% (n=2; 0·48 [0·26–0·87]). Overall, sanitation interventions reduced diarrhoea risk by 24% (0·76 [0·61–0·94]). Compared with unimproved sanitation, providing sewer connection reduced diarrhoea risk by 47% (n=5; 0·53 [0·30–0·93]). Promotion of handwashing with soap reduced diarrhoea risk by 30% (0·70 [0·64–0·76]).
Interpretation
WASH interventions reduced risk of diarrhoea in children in LMICs. Interventions supplying either water filtered at POU, higher water quality from an improved source on premises, or basic sanitation services with sewer connection were associated with increased reductions. Our results support higher service levels called for under SDG 6. Notably, no studies evaluated interventions that delivered access to safely managed WASH services, the level of service to which universal coverage by 2030 is committed under the SDG.
Related collections
Most cited references91

- Record: found
- Abstract: found
- Article: found
The PRISMA 2020 statement: an updated guideline for reporting systematic reviews

- Record: found
- Abstract: found
- Article: found
Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019

- Record: found
- Abstract: found
- Article: found