- Record: found
- Abstract: found
- Article: found
Quantifying the effects of temperature on mosquito and parasite traits that determine the transmission potential of human malaria
Read this article at
Abstract
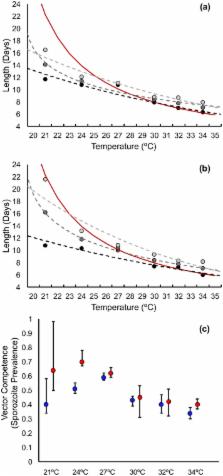
Malaria transmission is known to be strongly impacted by temperature. The current understanding of how temperature affects mosquito and parasite life history traits derives from a limited number of empirical studies. These studies, some dating back to the early part of last century, are often poorly controlled, have limited replication, explore a narrow range of temperatures, and use a mixture of parasite and mosquito species. Here, we use a single pairing of the Asian mosquito vector, An. stephensi and the human malaria parasite, P. falciparum to conduct a comprehensive evaluation of the thermal performance curves of a range of mosquito and parasite traits relevant to transmission. We show that biting rate, adult mortality rate, parasite development rate, and vector competence are temperature sensitive. Importantly, we find qualitative and quantitative differences to the assumed temperature-dependent relationships. To explore the overall implications of temperature for transmission, we first use a standard model of relative vectorial capacity. This approach suggests a temperature optimum for transmission of 29°C, with minimum and maximum temperatures of 12°C and 38°C, respectively. However, the robustness of the vectorial capacity approach is challenged by the fact that the empirical data violate several of the model’s simplifying assumptions. Accordingly, we present an alternative model of relative force of infection that better captures the observed biology of the vector–parasite interaction. This model suggests a temperature optimum for transmission of 26°C, with a minimum and maximum of 17°C and 35°C, respectively. The differences between the models lead to potentially divergent predictions for the potential impacts of current and future climate change on malaria transmission. The study provides a framework for more detailed, system-specific studies that are essential to develop an improved understanding on the effects of temperature on malaria transmission.
Author summary
Many of the mosquito and parasite life history traits that combine to influence the transmission intensity of malaria (e.g., adult mosquito longevity, biting rate, the developmental period of the parasite within the mosquito, and the proportion of mosquitoes that become infectious) are strongly temperature sensitive. Yet, in spite of decades of research, the precise relationships between individual traits and temperature remain poorly characterized. As a consequence, the majority of studies exploring the influence of local environmental conditions, or prospective impacts of climate change, draw on a combination of studies that utilize different experimental methods and a range of mosquito and parasite species. Here, we use the Indian malaria mosquito, Anopheles stephensi, and the human malaria parasite, Plasmodium falciparum, to thoroughly characterize the influence of temperature on key transmission-related traits. The results reveal a number of novel insights and challenge some longstanding assumptions regarding the nature of mosquito and parasite thermal responses. This study provides an experimental blueprint for further system-specific studies necessary to more fully understand the implications of changing temperatures on malaria transmission.
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
Drivers, dynamics, and control of emerging vector-borne zoonotic diseases.
- Record: found
- Abstract: found
- Article: not found
Optimal temperature for malaria transmission is dramatically lower than previously predicted.
- Record: found
- Abstract: found
- Article: not found