- Record: found
- Abstract: found
- Article: found
Rethinking the extrinsic incubation period of malaria parasites
Read this article at
Abstract
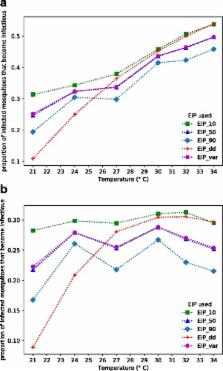
The time it takes for malaria parasites to develop within a mosquito, and become transmissible, is known as the extrinsic incubation period, or EIP. EIP is a key parameter influencing transmission intensity as it combines with mosquito mortality rate and competence to determine the number of mosquitoes that ultimately become infectious. In spite of its epidemiological significance, data on EIP are scant. Current approaches to estimate EIP are largely based on temperature-dependent models developed from data collected on parasite development within a single mosquito species in the 1930s. These models assume that the only factor affecting EIP is mean environmental temperature. Here, we review evidence to suggest that in addition to mean temperature, EIP is likely influenced by genetic diversity of the vector, diversity of the parasite, and variation in a range of biotic and abiotic factors that affect mosquito condition. We further demonstrate that the classic approach of measuring EIP as the time at which mosquitoes first become infectious likely misrepresents EIP for a mosquito population. We argue for a better understanding of EIP to improve models of transmission, refine predictions of the possible impacts of climate change, and determine the potential evolutionary responses of malaria parasites to current and future mosquito control tools.
Related collections
Most cited references90
- Record: found
- Abstract: found
- Article: not found
Impact of daily temperature fluctuations on dengue virus transmission by Aedes aegypti.
- Record: found
- Abstract: found
- Article: not found
Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing
- Record: found
- Abstract: found
- Article: not found