- Record: found
- Abstract: found
- Article: found
The Role of VP16 in the Life Cycle of Alphaherpesviruses
Read this article at
Abstract
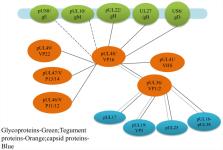
The protein encoded by the UL48 gene of alphaherpesviruses is named VP16 or alpha-gene-transactivating factor (α-TIF). In the early stage of viral replication, VP16 is an important transactivator that can activate the transcription of viral immediate-early genes, and in the late stage of viral replication, VP16, as a tegument, is involved in viral assembly. This review will explain the mechanism of VP16 acting as α-TIF to activate the transcription of viral immediate-early genes, its role in the transition from viral latency to reactivation, and its effects on viral assembly and maturation. In addition, this review also provides new insights for further research on the life cycle of alphaherpesviruses and the role of VP16 in the viral life cycle.
Related collections
Most cited references150
- Record: found
- Abstract: found
- Article: found
Tegument Assembly and Secondary Envelopment of Alphaherpesviruses
- Record: found
- Abstract: found
- Article: not found
The herpes simplex virus VP16-induced complex: the makings of a regulatory switch.
- Record: found
- Abstract: found
- Article: not found