- Record: found
- Abstract: not found
- Article: not found
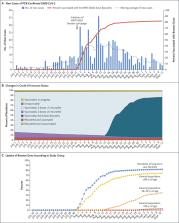
Effects of BNT162b2 Covid-19 Vaccine Booster in Long-Term Care Facilities in Israel
letter
Khitam Muhsen , Ph.D.
Nimrod Maimon , M.D., M.H.A.
Ami Mizrahi , L.L.B., M.Sc.,
Baruch Varticovschi ,
Omri Bodenheimer , M.Sc.
Udi Gelbshtein , M.H.A., P.A.-C.
Itamar Grotto , M.D., Ph.D.
Dani Cohen , Ph.D.
Ron Dagan , M.D.
22 December 2021
Keyword part (code): 15Keyword part (keyword): Geriatrics/AgingKeyword part (code): 15_1Keyword part (keyword): Geriatrics/Aging GeneralKeyword part (code): 15_5Keyword part (keyword): Rehabilitation ,
15,
Geriatrics/Aging,
Keyword part (code): 15_1Keyword part (keyword): Geriatrics/Aging GeneralKeyword part (code): 15_5Keyword part (keyword): Rehabilitation ,
15_1,
Geriatrics/Aging General,
15_5,
Rehabilitation,
Keyword part (code): 18Keyword part (keyword): Infectious DiseaseKeyword part (code): 18_2Keyword part (keyword): VaccinesKeyword part (code): 18_12Keyword part (keyword): Coronavirus ,
18,
Infectious Disease,
Keyword part (code): 18_2Keyword part (keyword): VaccinesKeyword part (code): 18_12Keyword part (keyword): Coronavirus ,
18_2,
Vaccines,
18_12,
Coronavirus,
Keyword part (code): 24Keyword part (keyword): Health PolicyKeyword part (code): 24_5Keyword part (keyword): Health Care Delivery ,
24,
Health Policy,
Keyword part (code): 24_5Keyword part (keyword): Health Care Delivery,
24_5,
Health Care Delivery
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Related collections
Most cited references4
- Record: found
- Abstract: found
- Article: not found
Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months
Einav Levin, Yaniv Lustig, Carmit Cohen … (2021)
- Record: found
- Abstract: found
- Article: not found
Protection of BNT162b2 Vaccine Booster against Covid-19 in Israel
Yinon Bar-On, Yair Goldberg, Micha Mandel … (2021)
- Record: found
- Abstract: found
- Article: not found
Nursing homes: the titanic of cruise ships – will residential aged care facilities survive the coronavirus disease 2019 pandemic?
Frances Crotty, Rosie Watson, Wen Kwang Lim (2020)
