- Record: found
- Abstract: found
- Article: found
Emerging therapeutic agents for advanced non-small cell lung cancer
Read this article at
Abstract
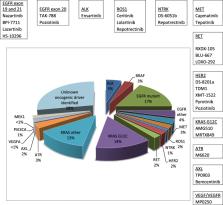
Advanced non-small cell lung cancer (NSCLC) is the most common type of lung cancer, with a poor prognosis and no known cure. Survival time is often short because of limited treatment options. Recent advances in targeted therapy and immunotherapy have changed the landscape for the treatment of advanced NSCLC. In the last 10 years, the US Food and Drug Administration (FDA) has approved more than 17 new medications for this devastating disease and more are coming. Molecular and immunogenic testing makes personalized medicine possible for patients with advanced NSCLC. The new medications provide promising efficacy and safety resulting in improved long-term survival for a significant number of patients. In this review, we summarize the recent advances in advanced/metastatic NSCLC therapeutics with a specific focus on first in-human or early-phase I/II clinical trials. These drugs either offer better alternatives to current standard drugs in the same class or are a completely new class of drugs with novel mechanisms of action. Advances are divided into (1) targeted agents, (2) antibody-drug conjugates, and (3) immunotherapies. Finally, we present a brief review of the emerging agents and ongoing clinical studies.
Related collections
Most cited references84
- Record: found
- Abstract: found
- Article: not found
Trastuzumab Deruxtecan in Previously Treated HER2-Positive Breast Cancer
- Record: found
- Abstract: found
- Article: not found
Brigatinib versus Crizotinib in ALK-Positive Non–Small-Cell Lung Cancer
- Record: found
- Abstract: found
- Article: not found