- Record: found
- Abstract: found
- Article: found
Schizotypy-Related Magnetization of Cortex in Healthy Adolescence Is Colocated With Expression of Schizophrenia-Related Genes
Abstract
Background
Genetic risk is thought to drive clinical variation on a spectrum of schizophrenia-like traits, but the underlying changes in brain structure that mechanistically link genomic variation to schizotypal experience and behavior are unclear.
Methods
We assessed schizotypy using a self-reported questionnaire and measured magnetization transfer as a putative microstructural magnetic resonance imaging marker of intracortical myelination in 68 brain regions in 248 healthy young people (14–25 years of age). We used normative adult brain gene expression data and partial least squares analysis to find the weighted gene expression pattern that was most colocated with the cortical map of schizotypy-related magnetization.
Results
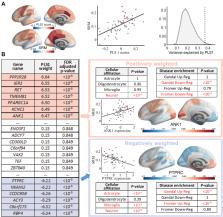
Magnetization was significantly correlated with schizotypy in the bilateral posterior cingulate cortex and precuneus (and for disorganized schizotypy, also in medial prefrontal cortex; all false discovery rate–corrected ps < .05), which are regions of the default mode network specialized for social and memory functions. The genes most positively weighted on the whole-genome expression map colocated with schizotypy-related magnetization were enriched for genes that were significantly downregulated in two prior case-control histological studies of brain gene expression in schizophrenia. Conversely, the most negatively weighted genes were enriched for genes that were transcriptionally upregulated in schizophrenia. Positively weighted (downregulated) genes were enriched for neuronal, specifically interneuronal, affiliations and coded a network of proteins comprising a few highly interactive “hubs” such as parvalbumin and calmodulin.
Related collections
Most cited references54
- Record: found
- Abstract: found
- Article: not found
BRAIN NETWORKS. Correlated gene expression supports synchronous activity in brain networks.
- Record: found
- Abstract: found
- Article: not found
Hierarchy of transcriptomic specialization across human cortex captured by structural neuroimaging topography
- Record: found
- Abstract: found
- Article: not found