- Record: found
- Abstract: found
- Article: found
A Tissue-Engineered Tracheobronchial In Vitro Co-Culture Model for Determining Epithelial Toxicological and Inflammatory Responses
Read this article at
Abstract
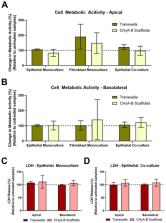
Translation of novel inhalable therapies for respiratory diseases is hampered due to the lack of in vitro cell models that reflect the complexity of native tissue, resulting in many novel drugs and formulations failing to progress beyond preclinical assessments. The development of physiologically-representative tracheobronchial tissue analogues has the potential to improve the translation of new treatments by more accurately reflecting in vivo respiratory pharmacological and toxicological responses. Herein, advanced tissue-engineered collagen hyaluronic acid bilayered scaffolds (CHyA-B) previously developed within our group were used to evaluate bacterial and drug-induced toxicity and inflammation for the first time. Calu-3 bronchial epithelial cells and Wi38 lung fibroblasts were grown on either CHyA-B scaffolds (3D) or Transwell ® inserts (2D) under air liquid interface (ALI) conditions. Toxicological and inflammatory responses from epithelial monocultures and co-cultures grown in 2D or 3D were compared, using lipopolysaccharide (LPS) and bleomycin challenges to induce bacterial and drug responses in vitro. The 3D in vitro model exhibited significant epithelial barrier formation that was maintained upon introduction of co-culture conditions. Barrier integrity showed differential recovery in CHyA-B and Transwell ® epithelial cultures. Basolateral secretion of pro-inflammatory cytokines to bacterial challenge was found to be higher from cells grown in 3D compared to 2D. In addition, higher cytotoxicity and increased basolateral levels of cytokines were detected when epithelial cultures grown in 3D were challenged with bleomycin. CHyA-B scaffolds support the growth and differentiation of bronchial epithelial cells in a 3D co-culture model with different transepithelial resistance in comparison to the same co-cultures grown on Transwell ® inserts. Epithelial cultures in an extracellular matrix like environment show distinct responses in cytokine release and metabolic activity compared to 2D polarised models, which better mimic in vivo response to toxic and inflammatory stimuli offering an innovative in vitro platform for respiratory drug development.
Related collections
Most cited references79
- Record: found
- Abstract: found
- Article: not found
Modeling Physiological Events in 2D vs. 3D Cell Culture
- Record: found
- Abstract: found
- Article: not found
TEER measurement techniques for in vitro barrier model systems.
- Record: found
- Abstract: found
- Article: found