- Record: found
- Abstract: found
- Article: found
Exploratory evaluation of an eye-tracking system in patients with advanced spinal muscular atrophy type I receiving nusinersen
Read this article at
Abstract
Objective
This study evaluated the feasibility of a matching-pair test using eye-tracking technology to assess nusinersen effectiveness in patients with advanced spinal muscular atrophy (SMA) type I.
Methods
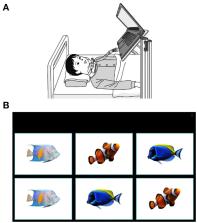
This prospective, observational study enrolled patients with 5q-SMA type I who had lost gross motor function. Three different levels of matching-pair tests were conducted using the eye-gaze system (My Tobii; TobiiDynavox Inc.) at baseline, and after 9 and 24 weeks of nusinersen treatment. The primary endpoint was the change from baseline in matching-pair test scores and response times (i.e., the time to answer matching-pair test) at 24 weeks from baseline. Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND), Pediatric Quality of Life inventory for patients with Neuromuscular Disease (PedsQL-NM) and Numerical Rating Scale (NRS) scores were also assessed as secondary endpoints. Analysis of ocular fixation was performed as an additional analysis. This study was registered at https://www.umin.ac.jp/ctr/ (UMIN000033935).
Results
Seven patients (one male, six female) aged 5–21 years (median 11 years) were enrolled; all patients were bedridden and six patients were ventilated. All seven patients were able to conduct level 1 matching-pair tests at each assessment; five patients were also able to conduct levels 2 and 3. Two patients (those with the highest CHOP-INTEND scores) were able to complete all tests correctly within 60 s. There was a non-significant trend toward improvement in CHOP-INTEND, PedsQL-NM, and NRS scores over the 6-month period. There were no significant differences in the number of actions, errors, correct answers, or response times between baseline and Week 9 or 24 at any level. However, the result of an additional analysis suggests that detection of eye movement would be useful to evaluate for advanced SMA.
Related collections
Most cited references41
- Record: found
- Abstract: found
- Article: not found
Nusinersen versus Sham Control in Infantile-Onset Spinal Muscular Atrophy

- Record: found
- Abstract: found
- Article: found
Spinal muscular atrophy
- Record: found
- Abstract: found
- Article: not found