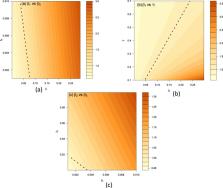
Introduction There have been more than twenty-five known outbreaks of Ebola virus disease in Africa since the disease was first identified in Zaire (now Democratic Republic of Congo) in 1976 (Centers for Disease Control and Prevention, 2014). Five ebolavirus strains have been identified in total, the most virulent of which appears to be the Ebola Zaire variant (EBOV); it was responsible for over a dozen outbreaks between 1976 and 2008, with overall case fatality rate of 79% (95% CI: 0.76–0.81) (Centers for Disease Control and Prevention, 2014; Breman et al., 1978; Formenty et al., 2003; Georges et al., 1999; Heymann et al., 1980; Khan et al., 1999; Leroy et al., 2004; Nkoghe et al., 2011; Pattyn, 1978; Report of an International Commission, 1978). Transmission occurs as a result of direct contact with the body fluids of infected individuals, and is unlikely to occur during the incubation period (Breman et al., 1978; Dowell et al., 1999). In March 2014, a new outbreak of EBOV was identified in West Africa. Cases were reported first in Guinea (Baize et al., 2014), then in Liberia, Sierra Leone, Nigeria and Senegal. The outbreak is the largest to date: as of 5th September 2014, 3944 cases have been reported by the World Health Organisation, and 1759 deaths (World Health Organisation, 2014). Unlike previous outbreaks, which were centred on rural communities, infections have also been detected in large urban areas in 2014. It is therefore crucial to develop a better understanding of the transmission dynamics of EBOV, and the implications it could have for control measures. There have been a number of modelling studies of Ebola, which have focused on two historical outbreaks (Table 1). For the 1995 outbreak in Democratic Republic of Congo, estimates of the basic reproduction number have ranged from 1.4 to 3.7 (Chowell et al., 2004; Ferrari et al., 2005; Legrand et al., 2007; Lekone and Finkenstädt, 2006; Ndanguza et al., 2013; White and Pagano, 2008); for the 2000/1 outbreak in Uganda, estimates span 1.3–2.7 (Chowell et al., 2004; Ferrari et al., 2005; Legrand et al., 2007; McKinley et al., 2009). These studies fitted models of varying complexity to time series with date of disease onset and/or death. However, in both outbreaks, hospital-based infection played a substantial role in transmission (Borchert et al., 2011; Khan et al., 1999; Francesconi et al., 2003). As the data were not stratified by likely source of infection, it was not possible to identify the relative contribution of different transmission routes to the reproduction number. It therefore remains unclear to what extent person-to-person transmission contributed to past Ebola outbreaks, and how community and hospital-specific control measures influenced the reproduction number in each setting. To gain further insights into the dynamics of Ebola, we revisited case data from the first known EBOV outbreak in 1976. These data included information on the likely source of infection, as well as date of onset and outcome. We used a transmission model to infer the basic reproduction number in different settings, and assessed the contribution of hospital and community infection to disease transmission. Having characterised the dynamics of EBOV, we used stochastic simulations to investigate alternative outcomes that could have been generated with the same epidemiological conditions present in 1976, and assessed the potential for a large outbreak of the disease. Finally, we discuss the implications of our results for other Ebola outbreaks. Methods Data Between August and November 1976, there were 318 reported cases of Ebola in the Yandongi collectivity of Zaire, with 280 deaths. The outbreak was centred around the Yambuku Mission Hospital. With only five syringes issued each day, exposure to contaminated syringes and needles during routine outpatient visits was a common route of transmission; infected hosts then returned to their villages, and in some cases infected others in the community (Breman et al., 1978). In our analysis, we used a line list of 262 cases, taken from the original epidemiological investigations (Breman et al., 1978; Report of an International Commission, 1978). The data (Supplementary File S1) reported: date of disease onset; outcome (death/recovery); date of outcome; and likely source of transmission (syringe during outpatient visit/person-to-person transmission/both/other). The progression of the outbreak is shown in Fig. 1. Of the reported 262 cases, 250 had a likely source of infection recorded and 8 dates of onset and outcome were missing (Table S1). We used the line list to compile four daily time series: onset of disease following hospital infection via syringe (87 cases in total); onset of disease following person-to-person infection (140 cases in total); reported deaths (248 cases in total); and reported recoveries (11 cases in total). Model We used a compartmental model of infection to analyse the temporal dynamics of Ebola (Legrand et al., 2007). The model structure is outlined in Fig. 2. We assumed that individuals start off susceptible to infection (S). Upon infection they enter an incubation period (E), then become symptomatic and infectious in the community (I). We therefore assume that the latent and incubation periods are equivalent. After this point, they either: enter a recovered state (R); remain infectious and go into hospital (H); or die and remain infectious (D) until buried (B). Following hospitalisation, infectious hosts also move either into the recovered or dead compartment. We assumed susceptible hosts in the community could become infected in three different ways: person-to-person transmission from an infectious host in the community, at rate β i (t), or from a dead but not buried patient during a traditional funeral ceremony, at rate β d (t); or hospital transmission via syringe during outpatient visits, at rate β h (t). There was evidence that hospital and person-to-person transmission declined over the course of the 1976 outbreak. Epidemiological reports note that the community stopped coming to the outpatient department as they associated the epidemic with the Yambuku Mission Hospital, which eventually was closed on 30th September. Also, as time went on the population became very suspicious and did not touch the corpses anymore, not even to bury them (Breman et al., 1978). We therefore used time-dependent smooth decreasing functions for β i (t), β d (t) and β h (t) (Chowell et al., 2004; Lekone and Finkenstädt, 2006; Ndanguza et al., 2013): (1) β i ( t ) = β i ( 1 − δ pp σ ( t , α pp , τ pp ) ) β d ( t ) = β d ( 1 − δ pp σ ( t , α pp , τ pp ) ) β h ( t ) = β h ( 1 − σ ( t , α h , τ h ) ) 1 t 1000 cases) to be around 3%. This means that given the same initial conditions, Ebola outbreaks would have been occasionally been large, just by chance. Moreover, a relatively high person-to-person transmission component (R 0pp ≈ 1) implied that the 1976 epidemic would have been difficult to control via hospital-based infection control measures alone. If the reduction in community transmission had been smaller, or infection had been seeded into a number of different communities, the outbreak could have continued for some time. Our results also suggest that changes in behaviour caused a significant reduction in both hospital-to-community and within-community transmission. Although Yambuku Mission hospital closed on the 30th September, we found that the reduction in transmission occurred well before this point, most likely from susceptible hosts having less contact with infected patients, and making fewer routine outpatient visits to the hospital (Breman et al., 1978). As well as contributing to transmission, infections from syringes also appeared to have a higher case fatality ratio (CFR) than person-to-person infections. This could have been the result of a larger viral inoculum during contact with a contaminated syringe. With more data on transmission events – including chains of person-to-person infection – it would be possible to further investigate the role of exposure in the natural history of Ebola infection. Even with four time series, it was not possible to robustly distinguish between person-to-person transmission resulting from contact with community cases and funeral attendance. Additional case data, such as dates on which patients took care of an infected case or attended a funeral ceremony could allow us to disentangle the relative role of these two routes of community transmission. However, it is plausible that individuals had similar contact rates with infected and dead patients. Epidemiological investigations in 1976 found that 86% of hosts infected from person-to-person transmission reported prior contact with alive Ebola patients; the same proportion reported attending the funeral of an infected case (Breman et al., 1978). Assuming similar transmissibility for both types of contact, this would be equivalent to setting β i = β d in our model. There are some additional limitations to the model. First, we assumed that hosts mixed randomly both in the community and hospital. This was a reasonable assumption given that we stratified the data by route of transmission and outcome. However, there was evidence that certain groups, such as women aged 15–29, were more likely to attend clinics at Yambuku Mission Hospital in 1976 and hence be exposed to syringes (Report of an International Commission, 1978). To model the dynamics of the infection at a finer resolution, for instance by comparing model outputs to age-stratified case data, it would be necessary to account for such heterogeneity. We also assumed that occurrence of reported cases was Poisson distributed, and the proportion reported did not vary over time or by location. This might be plausible when cases occur in a relatively short outbreak in a small geographic region, but during outbreaks that span a much larger geographic area and persist for several months, reporting could change with time and vary between different settings. Moreover, if the dynamics of Ebola were to be modelled in real-time, it would be important to account for potential delays in reporting of cases and outcomes. In our stochastic scenario analysis we also assumed that timing and magnitude of changes in transmission rate were independent of epidemic size. Our simulations that used parameters from the fitted model (Fig. 6A) therefore assumed that identification and control of the infection would not have occurred quicker if more individuals had been infected earlier. However, we tested the sensitivity of our results to timing of hospital closure by assuming that the hospital closed one week after the first case (Fig. 6C); we also explored the effects of a smaller change in magnitude in person-to-person risk (Fig. 66D). Ideally, it would be possible to define a functional relationship between incidence and changes in transmission rate (Funk et al., 2009). However, this relationship is likely to be complex and setting-specific: in 1976, behavioural changes reduced transmission (Breman et al., 1978); in other Ebola outbreaks, large amounts of infection have increased fear and mistrust in the community, which might also have increased transmission (Borchert et al., 2011; World Health Organisation, 2014). The modelling tools illustrated here could easily be adapted for other Ebola outbreaks, and highlight the benefits of having data on likely source of infection and time of onset, hospitalisation and outcome for each patient. Previous Ebola modelling studies have examined the 1995 outbreak in Kikwit, DRC (Chowell et al., 2004; Ferrari et al., 2005; Legrand et al., 2007; Lekone and Finkenstädt, 2006; White and Pagano, 2008; McKinley et al., 2009; Ndanguza et al., 2013), and the 2000/1 outbreak in Uganda (Chowell et al., 2004; Ferrari et al., 2005; Legrand et al., 2007). As in Yambuku in 1976, hospital-based transmission played a substantial role in both outbreaks (Khan et al., 1999; Francesconi et al., 2003). However, modelling studies so far have incorporated time series for onset and/or death only, which meant that it was not possible to robustly infer the role of different routes of infection, such as the contribution of hospital and community transmission. In contrast, by fitting a transmission model to time series stratified by transmission route, we were able to estimate the contribution of different sources of infection to the dynamics of the epidemic. We estimated that the overall R 0 was 4.71 (95% CI: 3.92–5.66) for the 1976 Yambuku outbreak. This is high compared to estimates of R 0 in the 1995 and 2000/1 outbreaks, which ranged from 1.34–3.65 (Table 1). However, our analysis suggests that most of the R 0 in 1976 consisted of transmission via syringe; the person-to-person basic reproduction number was 1.34 (0.92–2.11). Given data on likely source of infection in 1995 and 2000/1, it would be possible to establish whether person-to-person transmission contributed a similar amount to overall transmission during these outbreaks. Our estimate of a person-to-person basic reproduction number R 0pp ≈ 1 in 1976 suggests that Ebola would have been capable of generating a wide range of outbreak sizes in the absence of any extrinsic variation in epidemiological conditions. This implies that effective reduction in person-to-person transmission was crucial in reducing the potential size of the outbreak; stochastic simulations suggest Ebola could still have generated a large number of cases if hospital transmission was absent in 1976. Measures to reduce person-to-person transmission – including isolation of patients, follow-up surveillance of their contacts, and education to curtail infection in the community – are therefore likely to form a crucial part of the response to Ebola outbreaks (Borchert et al., 2011; Khan et al., 1999; Okware et al., 2002). As well as variation in social and cultural factors between different regions, the stochastic nature of Ebola outbreaks means that inference to other settings must be done with caution. Our analysis concentrates on a single outbreak of 318 cases, rather than a set of past Ebola outbreaks, which have ranged from a small number of cases to several thousand (Fig. 6B). Analyses of data from a large number of historical outbreaks simultaneously would help reduce this stochastic uncertainty and allow comparative studies to be performed. By making the line listing of the 1976 outbreak available (Supplementary File S1), we hope to stimulate such work. Comparative studies could potentially shed further light on which underlying factors contribute to the differences in outcome of Ebola outbreaks, and which control measures are likely to be most effective.