- Record: found
- Abstract: found
- Article: found
Sonochemical Synthesis of Silver Nanoparticles Using Starch: A Comparison
Read this article at
Abstract
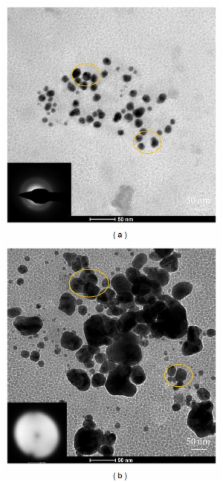
A novel approach was applied to synthesize silver nanoparticles using starch under sonication. Colloidal silver nanoparticles solution exhibited an increase of absorption from 420 to 440 nm with increase starch quantity. Transmission electron microscopy followed by selected area electron diffraction pattern analysis indicated the formation of spherical, polydispersed, amorphous, silver nanoparticles of diameter ranging from 23 to 97 nm with mean particle size of 45.6 nm. Selected area electron diffraction (SAED) confirmed partial crystalline and amorphous nature of silver nanoparticles. Silver nanoparticles synthesized in this manner can be used for synthesis of 2-aryl substituted benzimidazoles which have numerous biomedical applications. The optimized reaction conditions include 10 ml of 1 mM AgNO 3, 25 mg starch, 11 pH range, and sonication for 20 min at room temperature.
Related collections
Most cited references68
- Record: found
- Abstract: found
- Article: not found
A novel one-pot 'green' synthesis of stable silver nanoparticles using soluble starch.
- Record: found
- Abstract: not found
- Article: not found