- Record: found
- Abstract: found
- Article: not found
Drug Repurposing for Viral Infectious Diseases: How Far Are We?
Read this article at
Abstract
Despite the recent advances in controlling some viral pathogens, most viral infections still lack specific treatment. Indeed, the need for effective therapeutic strategies to combat ‘old’, emergent, and re-emergent viruses is not paralleled by the approval of new antivirals. In the past years, drug repurposing combined with innovative approaches for drug validation, and with appropriate animal models, significantly contributed to the identification of new antiviral molecules and targets for therapeutic intervention. In this review, we describe the main strategies of drug repurposing in antiviral discovery, discuss the most promising candidates that could be repurposed to treat viral infections, and analyze the possible caveats of this trendy strategy of drug discovery.
Highlights
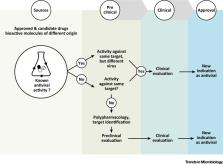
Repurposing existing drugs is an emerging strategy for expediting the approval of effective and safe therapeutics, such as for the treatment of orphan drug diseases.
New indications for antiviral activity can be identified for molecules of different origins showing repurposing potential by acting against a previously known target or a new antiviral target.
Innovative approaches for target validation (e.g., gene editing by CRISPR/Cas9) and new experimental models (e.g., organoids) allowed the identification of novel antiviral agents and the unraveling of molecular pathways underlying viral pathogenesis.
Drug repurposing has successfully identified promising candidate drugs that can open new therapeutic avenues to counteract current viral pathogens and possible emerging viruses.
Related collections
Most cited references55
- Record: found
- Abstract: found
- Article: not found
Identification of small-molecule inhibitors of Zika virus infection and induced neural cell death via a drug repurposing screen.
- Record: found
- Abstract: found
- Article: not found
Zika virus cell tropism in the developing human brain and inhibition by azithromycin.
- Record: found
- Abstract: found
- Article: not found
