- Record: found
- Abstract: found
- Article: found
Differential Electrophysiological Responses to Odorant Isotopologues in Drosophilid Antennae123
Read this article at
Abstract
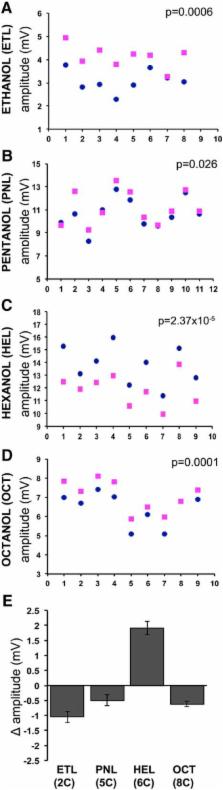
Olfaction presents the ultimate challenge to molecular recognition as thousands of molecules have to be recognized by far fewer olfactory receptors. We have presented evidence that Drosophila readily distinguish odorants based on their molecular vibrations using a battery of behavioral assays suggesting engagement of a molecular vibration-sensing component. Here we interrogate electrophysiologically the antennae of four Drosophilids and demonstrate conserved differential response amplitudes to aldehydes, alcohols, ketones, nitriles, and their deuterated isotopologues. Certain deuterated odorants evoked larger electroantennogram (EAG) amplitudes, while the response to the normal odorant was elevated in others. Significantly, benzonitrile isotopologues were not distinguishable as predicted. This suggests that isotopologue-specific EAG amplitudes result from differential activation of specific olfactory receptors. In support of this, odorants with as few as two deuteria evoke distinct EAG amplitudes from their normal isotopologues, and this is independent of the size of the deuterated molecule. Importantly, we find no evidence that these isotopologue-specific amplitudes depend on perireceptor mechanisms or other pertinent physical property of the deuterated odorants. Rather, our results strongly suggest that Drosophilid olfactory receptors are activated by molecular vibrations differentiating similarly sized and shaped odorants in vivo, yielding sufficient differential information to drive behavioral choices.
Related collections
Most cited references42
- Record: found
- Abstract: found
- Article: not found
Insect olfactory receptors are heteromeric ligand-gated ion channels.
- Record: found
- Abstract: found
- Article: not found
Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels.
- Record: found
- Abstract: found
- Article: not found