- Record: found
- Abstract: found
- Article: found
Upfront surgery for stage IIIA/B non-small cell lung cancer: retrospective cohort study
Read this article at
Abstract
Background
Stage III non-small cell lung cancer is a heterogeneous disease. Several international guidelines recommend neoadjuvant treatment before surgery; however, upfront surgery is the preferred approach for technically resectable non-small cell lung cancer in East Asia. The aim of this retrospective study was to evaluate the long-term outcomes of curative-intent upfront surgery in stage IIIA/B non-small cell lung cancer.
Methods
Patients who underwent curative-intent upfront surgery with stage cIIIA/B non-small cell lung cancer were identified. The clinical and pathological variables and survival outcomes were evaluated.
Results
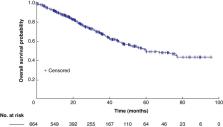
Overall, 664 patients were identified, of whom 320 (48.8%) had N2 disease, 66.7% were males, 49.4% had a smoking history, and 61.2% had lung adenocarcinoma. Lobectomy was the most performed surgical procedure (84.9%). A total of 40 patients (6.02%) had positive margins (R1/R2). The grade III adverse event rate was 2.0% (13 of 664). The median follow-up was 30.6 (range 1.9–97.7) months. At follow-up, the mortality rate was 13.3% (88 of 664) and 37.2% of patients (247 of 664) had recurrence. Lung (101 of 247 (40.9%)) and brain (53 of 247 (21.5%)) were the most common sites of recurrence. The median overall survival was 60.0 (95% c.i. 51.5 to 67.6) months, with overall survival probability at 1, 2, 3, and 5 years being 89.6%, 77.8%, 67.2%, and 49.0% respectively. The R0 cohort showed an improved median overall survival compared with the R1/R2 cohort (67.4 versus 26.5 months respectively; P = greater than 0.001). The multivariable analysis revealed that age greater than or equal to 65 years (HR 1.51, 95% c.i. 1.08 to 2.12; reference = age less than 65 years), tumour size (greater than or equal to 5 cm (HR 2.13, 95% c.i. 1.41 to 3.21) and greater than or equal to 3 cm but less than 5 cm (HR 1.15, 95% c.i. 0.78 to 1.71); reference = less than 3 cm), and adjuvant treatment (chemotherapy (HR 0.69, 95% c.i. 0.49 to 0.96) and targeted therapy (HR 0.30, 95% c.i. 0.12 to 0.76); reference = none) significantly predicted overall survival.
Abstract
This study provides important real-world data, with a sufficient sample size, that upfront surgery for stage IIIA/B non-small cell lung cancer could have an acceptable perioperative morbidity rate and favourable long-term survival, particularly when combined with adjuvant therapies. This study identifies specific patient characteristics that may make them more suitable candidates for upfront surgery, such as younger age, smaller tumour size, limited nodal involvement, and no distant metastases. A comprehensive literature review on the survival rates of upfront surgery in non-small cell lung cancer facilitates easy comparison with relevant research findings in this field.
Related collections
Most cited references79
- Record: found
- Abstract: found
- Article: not found
Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer.
- Record: found
- Abstract: found
- Article: not found
Osimertinib in Resected EGFR-Mutated Non–Small-Cell Lung Cancer
- Record: found
- Abstract: found
- Article: not found