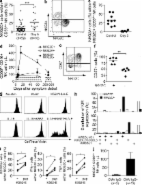
Several experimental models have demonstrated a role for NK cells in host responses against virus infections (Lodoen and Lanier, 2006; Lee et al., 2007). The perhaps most well characterized experimental model system in this respect is that of infection of mice with mouse CMV (Dokun et al., 2001; Lodoen and Lanier, 2006; Sun et al., 2009). In experimental mouse CMV infection, the NK cell response is characterized by proliferation of a specific subset of NK cells that peaks within a few days after infection. Subsequently, this NK cell population undergoes rapid contraction by apoptosis (Dokun et al., 2001; Robbins et al., 2004). To investigate more directly how results from studies of viral infections in experimental model systems compare with infections in humans, we have studied the NK cell response throughout the course of an acute virus infection in humans. In humans, involvement of NK cells in host responses to viruses were first indicated by the finding that virus-induced IFN-α enhanced NK cell–mediated cytotoxicity (Santoli et al., 1978; Trinchieri et al., 1978). Subsequently, low NK cell cytotoxic activity was linked to increased sensitivity to severe disseminating herpesvirus infections (Ching and Lopez, 1979; Quinnan et al., 1982; Merino et al., 1986; Joncas et al., 1989). NK cell defects were also shown to occur at chronic stages of HIV infection (Bonavida et al., 1986; Katz et al., 1987). Perhaps the most convincing data, however, for a role of NK cells in host responses to viral infections in humans has come from studies of patients with primary immunodeficiencies affecting NK cell numbers and/or NK cell function (Biron et al., 1989; Orange, 2006; Bryceson et al., 2007). In addition, several studies have described different characteristics of NK cells in patients with chronic viral infections (Fauci et al., 2005; Rehermann and Nascimbeni, 2005). However, few studies have more directly followed the human NK cell response throughout an acute virus infection. The opportunity to do so accompanied a Puumala hantavirus outbreak that occurred in Northern Sweden during 2007 (Pettersson et al., 2008). In humans, Puumala hantaviruses cause hemorrhagic fever with renal syndrome, a disease characterized by severe symptoms with occasional mortalities which stem from capillary leakage (Vapalahti et al., 2003; Schönrich et al., 2008). In infected individuals, virus replication has been documented in vascular endothelium, but the virus does not seem to cause direct cytopathic effects (Schönrich et al., 2008). The ensuing viremia that develops is normally cleared within 1–2 wk after the onset of symptoms (Schönrich et al., 2008). During the course of the present Puumala hantavirus outbreak, we prospectively collected clinical samples and followed NK cell responses in 16 patients from their first presentation at the emergency unit with acute symptoms until up to 15 mo after symptom debut. This enabled us to investigate in detail the NK cell response in virally infected humans, from the very first days of clinical symptoms until resolution of disease and beyond. The results show that NK cells, in a majority of the studied patients, rapidly expand and remain at significantly elevated numbers for >2 mo thereafter. Possible mechanisms behind this finding were investigated and the functionality of responding cells was determined. The results are discussed in relation to NK cell memory and the possible role of previous virus infections the present responses. RESULTS AND DISCUSSION NK cells rapidly expand and persist at elevated levels during acute hantavirus infection in humans In blood samples from 16 patients infected with hantavirus (Table S1 and Fig. S1), absolute numbers of total lymphocytes, total NK cells, and individual NK cell subsets were determined at days 5 and 60 after the onset of symptoms. Surprisingly, at day 60 after onset of symptoms, total lymphocyte numbers were increased approximately twofold compared with those on day 5 (Fig. 1 a). Among lymphocytes, NK cell numbers increased three- to fourfold (Fig. 1, b and c). When specific NK cell subsets were studied, the numbers of CD56dim and atypical CD56neg (Gonzalez et al., 2009; Björkström et al., 2010b) NK cells had increased markedly, whereas the numbers of CD56bright NK cells did not change significantly (Fig. 1, d and e). The increases in absolute numbers of NK cells correlated well with heightened frequencies of total NK cells (Fig. S2 a) and specific NK cell subpopulations (Fig. S2 b). Figure 1. Increase in CD56dim NK cells in human hantavirus infection. PBMCs from patients with acute hantavirus infection were analyzed by flow cytometry. (a and b) Absolute numbers of lymphocytes (a) and NK cells (b) at days 5 and 60 after symptom debut. (c) Relative changes of total lymphocytes and NK cells from days 5–60 after symptom debut (mean ± SEM). (d) Definition of CD56brightCD16− (i), CD56brightCD16+ (ii), CD56dimCD16+ (iii), and CD56−CD16+ (iv) NK cells by flow cytometry from one representative patient with acute hantavirus infection. Cells were gated on the CD3−CD4−CD14−CD19− population within the single cell lymphocyte gate. (e) Absolute numbers of the different NK cell subsets at days 5 and 60. For a–e, n = 16. *, P 60 d. Consistent with previous reports on NK cell memory-like features in mice (O’Leary et al., 2006; Sun et al., 2009), one may speculate that some NKG2C+ NK cells in CMV-positive individuals harbor such features and are among the cells that rapidly proliferate in response to hantavirus infection. The present observations suggest that the human NK cell population inherently may possess features not classically attributed to the innate immune response (Sun and Lanier, 2009), including long-term persistence of specific subsets of cells and, possibly, memory-like features. Whether this represents an adaptation of the NK cell repertoire to future infections with the same or similar pathogens is unclear. Given the fact that NK cells are parts of the innate immune system, these findings merit redefinition of the possible features of an innate immune response. MATERIALS AND METHODS Study design and human material. Peripheral blood was prospectively obtained from 16 patients infected with hantavirus. The following inclusion criteria were used: (a) Verified diagnosis of acute hantavirus infection. Infection was verified by an immunofluorescence test for hantavirus-reactive IgM and IgG antibodies in sera from the patients or viral load quantification by real-time PCR from patient plasma as previously described (Evander et al., 2007). (b) Access to a first sample drawn at an early time point after symptom debut (typically 3–5 d). (c) Sequential acquisition of peripheral blood during acute and convalescent phases of infection according to a defined sampling schedule with weekly samples taken during the first three weeks and later follow up samples. 35 uninfected blood donors, age- and sex-matched with the infected patients, were included as a control cohort. For isolation of PBMC, whole blood from infected patients was collected in CPT tubes (BD), centrifuged, and washed. PBMCs were frozen in 90% human albumin (Octapharma), 10% DMSO (WAK-Chemie Medical), and 50 IE heparin (LEO Pharma) and stored at −150°C for later analysis. The study was approved by the Regional Ethics Committee of Umeå University (approval number 04-113M). Written and oral informed consent was obtained from all study subjects. All clinical data, including lymphocyte count, were obtained through standard clinical procedures. Antibodies for flow cytometry. The following mAbs were used: anti-CD3 Pacific blue and anti-CD3 Cascade yellow (Dako); anti-CD56 PE-Cy7, anti-CD14 APC-Cy7, anti-CD16 Pacific blue, anti-CD4 biotin, anti-Ki67 FITC, anti-Bcl-2 PE, anti–ICAM-1 PE, anti-KIR3DL1 (Dx9 clone) FITC, anti-CD107a FITC, and anti-CD19 APC-Cy7 (BD); anti-CD4 biotin, visualized with Streptavidin Qdot 605 (Invitrogen); anti-KIR3DL1 (Dx9 clone) Alexa Fluor 700 (BioLegend); anti-NKG2A, anti-KIR2DL1/S1 (EB6 clone) APC, and anti-CD155 (Beckman Coulter); anti-NKG2A, conjugated with Pacific blue using a mAb labeling kit (Invitrogen); anti-KIR2DL2/S2/2DL3 (Gl183 clone; Beckman Coulter), biotinylated with FluoReporter Mini-Biotin-XX Protein Labeling kit (Invitrogen) and detected with Streptavidin PerCP (BD); anti-NKG2C PE, anti-MICA, anti-MICB, anti-ULBP1, anti-ULBP2, anti-ULBP3, anti-ULBP4, anti-KIR2DL3 (180701 clone) FITC, and anti-KIR2DL1 (143211 clone) FITC (R&D Systems); anti–HLA-E (eBioscience); and anti-CD112 (RDI). Intracellular cytokines were visualized with anti-TNF Alexa Fluor 647 (eBioscience) and anti–IFN-γ FITC (BD). HLA-A2 expression was evaluated with anti–HLA-A2 PE (clone BB7.2; BD) and HLA-C expression was evaluated with the L31 hybridoma (provided by L. Berg, Karolinska Insitutet, Stockholm, Sweden) after acid-wash treatment of the cells. Unconjugated mABs were visualized using a secondary APC mAB (BD). CD8 T cells specific for CMV were identified and enumerated using APC-conjugated HLA-A2 tetrameric complexes refolded with the CMV pp65 epitope NLVPMVATV (Beckman Coulter). Flow cytometry. Cell surface staining of purified PBMC or HUVECs (human umbilical cord endothelial cells; Lonza) was performed as previously described (Björkström et al., 2010a). For intracellular staining of PBMC with anti-Ki67, anti–BCL-2, or cytokines, cells were permeabilized with Cytofix/Cytoperm (BD). Samples were acquired on a CyAn ADP nine-color flow cytometer (Beckman Coulter) equipped with a 25-mW 405-nm laser, a 20-mW 488-nm laser, and a 25-mW 635-nm laser as previously described (Björkström et al., 2010a). Single-stained polystyrene beads (BD) were used for compensation purposes. Software-based compensation was performed using the compensation platform in FlowJo software version 8 (Tree Star, Inc.). Infection of endothelial cells. Pooled HUVECs were grown according to the manufacturer’s instructions using EGM-2 BulletKit (Lonza). Before infections, cells were seeded in cell culture plates and grown without supplementing the EGM-2 medium with hydrocortisone until 90% confluency. The Hantaan hantavirus (HTNV) strain 76–118 was used in the present study. Propagation and titration of HTNV were performed on Vero E6 cells as previously described (Stoltz et al., 2007). Cells were infected, or treated with the same amount of UV-inactivated virus as a control for nonreplicating virus, or with medium alone as a negative control. Detection of virus-infected cells. At 24, 48, 72, and 96 h after HTNV infection, HUVECs were fixed in methanol for 10 min at room temperature, followed by an incubation for 1 h at 37°C with convalescent human anti-hantavirus serum diluted 1:40 in PBS. After rinsing three times with PBS, cells were incubated for 1 h at 37°C with FITC-conjugated goat anti–human IgG (Sigma-Aldrich) diluted 1:50 and 5 µg/ml DAPI (Sigma-Aldrich) in PBS. KIR and HLA genotyping. Genomic DNA was isolated from 100 µl of peripheral blood using DNase Blood and Tissue KIT (QIAGEN). KIR genotyping was done as previously described using PCR-SPP technology and a KIR typing kit (Olerup SPP; Fauriat et al., 2008). The KIR ligand -Bw4, -Cw3 (C1), and -Cw4 (C2) motifs were determined using the KIR HLA ligand kit (Olerup-SPP). Cell lines and surface stabilization of HLA-E. K562 cells transfected with HLA-E*01033 (K562-E; provided by K. Söderström, Novo Nordisk A/S, Copenhagen, Denmark) were maintained in RPMI 1640 medium supplemented with 100 µg/ml l-glutamine, 10% heat-inactivated FCS, 100 U/ml penicillin G, 100 µg/ml streptomycin, and 1 mg/ml geneticin. Before functional experiments with K562-E cells, HLA-E expression was stabilized by pulsing with 100 µM of the synthetic HLA-G*0101 signal peptide VMAPRTLFL at 26°C and 5% CO2 for 15 h. NK cell functional assays. PBMCs were thawed and rested overnight in complete medium at a concentration of 106 cells/ml in 37°C and 5% CO2. The next day, 0.2 × 106 PBMCs were mixed with target cells at a ratio of 10:1 in V-bottom 96-well plates in a final volume of 200 µl and incubated for 6 h at 37°C in 5% CO2. When intracellular cytokine staining was performed, Brefeldin A (GolgiPlug; BD) was included at a dilution of 1:250 after 1 h of co-culture. After incubation, cells were surface stained and evaluated for CD107a, IFN-γ, and TNF expression as previously described (Bryceson et al., 2010). NK cell proliferation assay. To assess cytokine and/or HLA-E–induced proliferation of NKG2C+ cells, NK cells were isolated from PBMC of healthy individuals using an NK cell isolation kit (Miltenyi Biotec), labeled with CellTrace violet (Invitrogen), and incubated for 7 d with irradiated (90 Gy) K562 cells or K562*HLA-E cells (NK cell to target cell ratio of 1:1) in the presence or absence of 20 ng/ml human recombinant IL-15 (PeproTech). Proliferation was assessed by analyzing dilution of CellTrace violet in NKG2C+ CD56dim NK cells by flow cytometry. The K562*HLA-E transfectant (clone 2B4), constitutively expressing stabilized HLA-E (Falk et al., 2002), was provided by C.S. Falk (University of Heidelberg, Heidelberg, Germany). Statistics. Data were statistically analyzed using Prism software (GraphPad Software, Inc.). P-values of 15 observations, parametric statistical tests were used, for example, paired and nonpaired Student’s t tests. If nothing else is noted, bars in the figures represent SEM. Online supplemental material. Fig. S1 shows clinical and virological data from the hantavirus-infected patients. Fig. S2 shows the relative frequency of NK cells of total lymphocytes in the infected patients and healthy controls, as well as the relative frequencies of CD56brightCD16−, CD56brightCD16+, CD56dimCD16+, and CD56−CD16+ NK cells out of total NK cells in the infected patients and healthy controls. Fig. S3 presents data on the levels of NK cell stimulatory cytokines from the infected patients. Fig. S4 shows the FACS gating algorithm used to identify single-, double-, and triple-KIR+ NK cells and representative FACS plots for the algorithm used to dissect expression of KIR2DL1 and KIR2DS1, as well as KIR2DL3 and KIR2DL2/S2 on NK cells. Table S1 shows clinical characteristics of patients included in the study. Table S2 shows KIR and KIR-ligand genotyping of infected patients. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20100762/DC1.