- Record: found
- Abstract: found
- Article: found
Multiparameter Continuous Physiological Monitoring Technologies in Neonates Among Health Care Providers and Caregivers at a Private Tertiary Hospital in Nairobi, Kenya: Feasibility, Usability, and Acceptability Study
Abstract
Background
Continuous physiological monitoring technologies are important for strengthening hospital care for neonates, particularly in resource-constrained settings, and understanding user perspectives is critical for informing medical technology design, development, and optimization.
Objective
This study aims to assess the feasibility, usability, and acceptability of 2 noninvasive, multiparameter, continuous physiological monitoring technologies for use in neonates in an African health care setting.
Methods
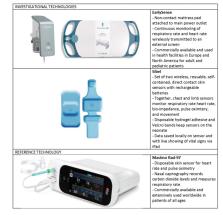
We assessed 2 investigational technologies from EarlySense and Sibel, compared with the reference Masimo Rad-97 technology through in-depth interviews and direct observations. A purposive sample of health care administrators, health care providers, and caregivers at Aga Khan University Hospital, a tertiary, private hospital in Nairobi, Kenya, were included. Data were analyzed using a thematic approach in NVivo 12 software.
Results
Between July and August 2020, we interviewed 12 health care providers, 5 health care administrators, and 10 caregivers and observed the monitoring of 12 neonates. Staffing and maintenance of training in neonatal units are important feasibility considerations, and simple training requirements support the feasibility of the investigational technologies. Key usability characteristics included ease of use, wireless features, and reduced number of attachments connecting the neonate to the monitoring technology, which health care providers considered to increase the efficiency of care. The main factors supporting acceptability included caregiver-highlighted perceptions of neonate comfort and health care respondent technology familiarity. Concerns about the side effects of wireless connections, electromagnetic fields, and mistrust of unfamiliar technologies have emerged as possible acceptability barriers to investigational technologies.
Conclusions
Overall, respondents considered the investigational technologies feasible, usable, and acceptable for the care of neonates at this health care facility. Our findings highlight the potential of different multiparameter continuous physiological monitoring technologies for use in different neonatal care settings. Simple and user-friendly technologies may help to bridge gaps in current care where there are many neonates; however, challenges in maintaining training and ensuring feasibility within resource-constrained health care settings warrant further research.
Related collections
Most cited references18
- Record: found
- Abstract: not found
- Article: not found
Using thematic analysis in psychology
- Record: found
- Abstract: found
- Article: not found
Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups.

- Record: found
- Abstract: found
- Article: found