- Record: found
- Abstract: found
- Article: found
Long-term open-label vebicorvir for chronic HBV infection: Safety and off-treatment responses
Read this article at
Abstract
Background & Aims
The investigational first-generation core inhibitor vebicorvir (VBR) demonstrated safety and antiviral activity over 24 weeks in two phase IIa studies in patients with chronic HBV infection. In this long-term extension study, patients received open-label VBR with nucleos(t)ide reverse transcriptase inhibitors (NrtIs).
Methods
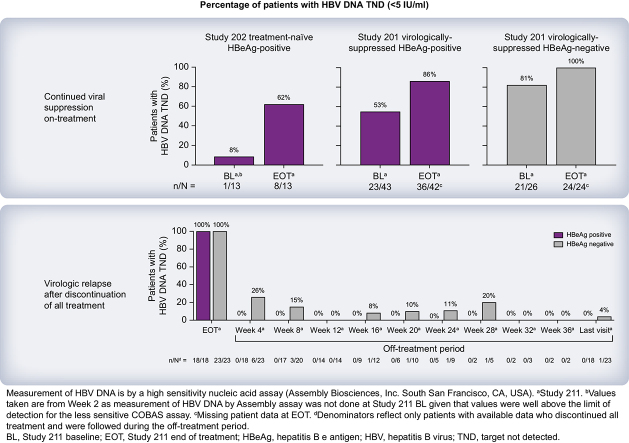
Patients in this study (NCT03780543) previously received VBR + NrtI or placebo + NrtI in parent studies 201 (NCT03576066) or 202 (NCT03577171). After receiving VBR + NrtI for ≥52 weeks, stopping criteria (based on the treatment history and hepatitis B e antigen status in the parent studies) were applied, and patients either discontinued both VBR + NrtI, discontinued VBR only, or continued both VBR + NrtI. The primary efficacy endpoint was the proportion of patients with HBV DNA <20 IU/ml at 24 weeks off treatment.
Results
Ninety-two patients entered the extension study and received VBR + NrtI. Long-term VBR + NrtI treatment led to continued suppression of HBV nucleic acids and, to a lesser extent, HBV antigens. Forty-three patients met criteria to discontinue VBR + NrtI, with no patients achieving the primary endpoint; the majority of virologic rebound occurred ≥4 weeks off treatment. Treatment was generally well tolerated, with few discontinuations due to adverse events (AEs). There were no deaths. Most AEs and laboratory abnormalities were related to elevations in alanine aminotransferase and occurred during the off-treatment or NrtI-restart phases. No drug–drug interactions between VBR + NrtI and no cases of treatment-emergent resistance among patients who adhered to treatment were observed.
Conclusions
Long-term VBR + NrtI was safe and resulted in continued reductions in HBV nucleic acids following completion of the 24-week parent studies. Following treatment discontinuation, virologic relapse was observed in all patients. This first-generation core inhibitor administered with NrtI for at least 52 weeks was not sufficient for HBV cure.
Impact and implications
Approved treatments for chronic hepatitis B virus infection (cHBV) suppress viral replication, but viral rebound is almost always observed after treatment discontinuation, highlighting an unmet need for improved therapies with finite treatment duration producing greater therapeutic responses that can be sustained off treatment. First-generation core inhibitors, such as vebicorvir, have mechanisms of action orthogonal to standard-of-care therapies that deeply suppress HBV viral replication during treatment; however, to date, durable virologic responses have not been observed after treatment discontinuation. The results reported here will help researchers with the design and interpretation of future studies investigating core inhibitors as possible components of finite treatment regimens for patients with cHBV. It is possible that next-generation core inhibitors with enhanced potency may produce deeper and more durable antiviral activity than first-generation agents, including vebicorvir.
Graphical abstract
Highlights
-
•
The HBV core inhibitor vebicorvir (VBR) + NrtI was evaluated in phase II studies.
-
•
Long-term VBR + NrtI is generally well tolerated in patients with chronic HBV infection.
-
•
Reductions in HBV DNA and pgRNA were observed with long-term VBR + NrtI treatment.
-
•
In patients who stopped treatment, a sustained virologic response was not observed.
-
•
Drug–drug interactions and viral resistance were not observed in this study.
Related collections
Most cited references15
- Record: found
- Abstract: found
- Article: not found
EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection.
- Record: found
- Abstract: found
- Article: not found
Peginterferon Alfa-2a, lamivudine, and the combination for HBeAg-positive chronic hepatitis B.
- Record: found
- Abstract: found
- Article: not found