- Record: found
- Abstract: found
- Article: found
Light-induced formation of partially reduced oxygen species limits the lifetime of photosystem 1-based biocathodes
Read this article at
Abstract
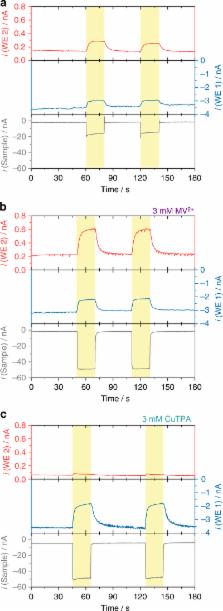
Interfacing photosynthetic proteins specifically photosystem 1 (PS1) with electrodes enables light-induced charge separation processes for powering semiartificial photobiodevices with, however, limited long-term stability. Here, we present the in-depth evaluation of a PS1/Os-complex-modified redox polymer-based biocathode by means of scanning photoelectrochemical microscopy. Focalized local illumination of the bioelectrode and concomitant collection of H 2O 2 at the closely positioned microelectrode provide evidence for the formation of partially reduced oxygen species under light conditions. Long-term evaluation of the photocathode at different O 2 concentrations as well as after incorporating catalase and superoxide dismutase reveals the particularly challenging issue of avoiding the generation of reactive species. Moreover, the evaluation of films prepared with inactivated PS1 and free chlorophyll points out additional possible pathways for the generation of oxygen radicals. To avoid degradation of PS1 during illumination and hence to enhance the long-term stability, the operation of biophotocathodes under anaerobic conditions is indispensable.
Abstract
Photobiodevices use photosynthetic proteins such as those of the photosystem 1 (PS1) to enable light-induced charge separation, but they suffer from limited long-term stability. Here authors employ scanning photoelectrochemical microscopy on a PS1 biocathode and find that several pathways generate oxygen radicals.
Related collections
Most cited references50
- Record: found
- Abstract: found
- Article: not found
Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants.
- Record: found
- Abstract: found
- Article: not found
Photoinhibition of photosystem II under environmental stress.
- Record: found
- Abstract: found
- Article: not found