- Record: found
- Abstract: found
- Article: not found
Telmisartan for treatment of Covid-19 patients: An open multicenter randomized clinical trial
Read this article at
Abstract
Background
Angiotensin receptor blockers (ARBs), such as telmisartan, have been postulated to treat Covid-19-induced lung inflammation.
Methods
This is a parallel-group, randomized, two-arm, open-label, adaptive, multicenter superiority trial with 1:1 allocation ratio. Participants included patients from 18 years of age hospitalized with Covid-19 with 4 or fewer days since symptom onset enrolled at a university and a community hospital in Buenos Aires, Argentina. Exclusion criteria included prior intensive care unit (ICU) admission and use of ARBs/angiotensin converting enzyme inhibitors at randomization . Control arm received standard care alone and treatment arm telmisartan 80 mg twice daily for 14 days. Primary outcomes were C-reactive protein (CRP) plasma levels at day 5 and 8 after randomization. Secondary outcomes included time to discharge within 15 days, admission to ICU and death at 15- and 30-days. NCT04355936 (Completed).
Findings
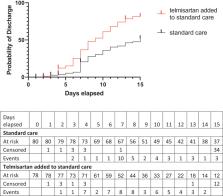
A pragmatic decision to end the study before the third interim analysis was made on Oct. 30th due to sharp reduction in recruitment. A total of 162 patients were randomized. 158 patients enrolled between May 14 and October 30 2020, were included in the analysis, 80 in the standard care and 78 in the telmisartan added to standard care group. Baseline absolute CRP serum levels were 5.53 ± 6.19 mg/dL (95% CI 6.91 to 4.15, n = 80) and 9.04 ± 7.69 (95% CI 9.04 to 10.82, n = 74) in the standard care and telmisartan added to standard care groups, respectively. Day 5 control-group CRP levels were 6.06 ± 6.95 mg/dL (95% CI 7.79–4.35, n = 66) while telmisartan group were 3.83 ± 5.08 mg/dL (95% CI 5.08–2.59, n = 66, p = 0.038). Day 8 CRP levels were 6.30 ± 8.19 mg/dL (95% CI 8.79–3.81, n = 44) and 2.37 ± 3.47 mg/dL (95% CI 3.44–1.30, n = 43, p = 0.0098) in the control and telmisartan groups, respectively (all values expressed as mean ± SD). Kaplan-Meier analysis showed that telmisartan-treated patients had a lower median time-to-discharge (control=15 days; telmisartan=9 days). Death by day 30 was reduced in the telmisartan-treated group (control 22.54%, 16/71; telmisartan 4.29%, 3/70 participants; p = 0.0023). Composite ICU, mechanical ventilation or death was reduced by telmisartan treatment at days 15 and 30. No adverse events were reported.
Interpretation
Our study suggests that the ARB telmisartan, a widely used antihypertensive drug, is safe and could reduce morbidity and mortality in hospitalized patients infected with SARS -CoV-2 by anti-inflammatory effects. Further studies employing telmisartan are needed for confirmation of our results and to define its true therapeutic value as a tool against Covid-19.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Receptor Recognition by the Novel Coronavirus from Wuhan: an Analysis Based on Decade-Long Structural Studies of SARS Coronavirus
- Record: found
- Abstract: found
- Article: not found
Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury

- Record: found
- Abstract: found
- Article: found
