- Record: found
- Abstract: found
- Article: found
Confidence and Receptivity for COVID-19 Vaccines: A Rapid Systematic Review
Read this article at
Abstract
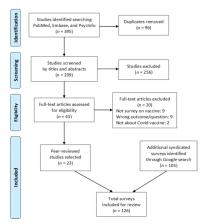
While COVID-19 continues raging worldwide, effective vaccines are highly anticipated. However, vaccine hesitancy is widespread. Survey results on uptake intentions vary and continue to change. This review compared trends and synthesized findings in vaccination receptivity over time across US and international polls, assessing survey design influences and evaluating context to inform policies and practices. Data sources included academic literature (PubMed, Embase, and PsycINFO following PRISMA guidelines), news and official reports published by 20 October 2020. Two researchers independently screened potential peer-reviewed articles and syndicated polls for eligibility; 126 studies and surveys were selected. Declining vaccine acceptance (from >70% in March to <50% in October) with demographic, socioeconomic, and partisan divides was observed. Perceived risk, concerns over vaccine safety and effectiveness, doctors’ recommendations, and inoculation history were common factors. Impacts of regional infection rates, gender, and personal COVID-19 experience were inconclusive. Unique COVID-19 factors included political party orientation, doubts toward expedited development/approval process, and perceived political interference. Many receptive participants preferred to wait until others have taken the vaccine; mandates could increase resistance. Survey wording and answer options showed influence on responses. To achieve herd immunity, communication campaigns are immediately needed, focusing on transparency and restoring trust in health authorities.
Related collections
Most cited references100
- Record: found
- Abstract: found
- Article: not found
The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration
- Record: found
- Abstract: found
- Article: not found
A global survey of potential acceptance of a COVID-19 vaccine
- Record: found
- Abstract: found
- Article: not found