- Record: found
- Abstract: found
- Article: found
Labile iron in cells and body fluids: physiology, pathology, and pharmacology
Read this article at
Abstract
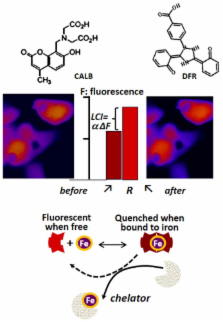
In living systems iron appears predominantly associated with proteins, but can also be detected in forms referred as labile iron, which denotes the combined redox properties of iron and its amenability to exchange between ligands, including chelators. The labile cell iron (LCI) composition varies with metal concentration and substances with chelating groups but also with pH and the medium redox potential. Although physiologically in the lower μM range, LCI plays a key role in cell iron economy as cross-roads of metabolic pathways. LCI levels are continually regulated by an iron-responsive machinery that balances iron uptake versus deposition into ferritin. However, LCI rises aberrantly in some cell types due to faulty cell utilization pathways or infiltration by pathological iron forms that are found in hemosiderotic plasma. As LCI attains pathological levels, it can catalyze reactive O species (ROS) formation that, at particular threshold, can surpass cellular anti-oxidant capacities and seriously damage its constituents. While in normal plasma and interstitial fluids, virtually all iron is securely carried by circulating transferrin (Tf; that renders iron essentially non-labile), in systemic iron overload (IO), the total plasma iron binding capacity is often surpassed by a massive iron influx from hyperabsorptive gut or from erythrocyte overburdened spleen and/or liver. As plasma Tf approaches iron saturation, labile plasma iron (LPI) emerges in forms that can infiltrate cells by unregulated routes and raise LCI to toxic levels. Despite the limited knowledge available on LPI speciation in different types and degrees of IO, LPI measurements can be and are in fact used for identifying systemic IO and for initiating/adjusting chelation regimens to attain full-day LPI protection. A recent application of labile iron assay is the detection of labile components in intravenous iron formulations per se as well as in plasma (LPI) following parenteral iron administration.
Related collections
Most cited references61
- Record: found
- Abstract: found
- Article: not found
Non-transferrin bound iron: a key role in iron overload and iron toxicity.
- Record: found
- Abstract: not found
- Article: not found
Iron overload in human disease.
- Record: found
- Abstract: found
- Article: not found