- Record: found
- Abstract: found
- Article: found
Factors influencing the participation of gastroenterologists and hepatologists in clinical research
Read this article at
Abstract
Background
Although clinical research is integral to the advancement of medical knowledge, physicians face a variety of obstacles to their participation as investigators in clinical trials. We examined factors that influence the participation of gastroenterologists and hepatologists in clinical research.
Methods
We surveyed 1050 members of the American Association for the Study of Liver Diseases regarding their participation in clinical research. We compared the survey responses by specialty and level of clinical trial experience.
Results
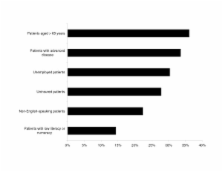
A majority of the respondents (71.6%) reported involvement in research activities. Factors most influential in clinical trial participation included funding and compensation (88.3%) and intellectual pursuit (87.8%). Barriers to participation were similar between gastroenterologists (n = 160) and hepatologists (n = 189) and between highly experienced (n = 62) and less experienced (n = 159) clinical researchers. These barriers included uncompensated research costs and lack of specialized support. Industry marketing was a greater influence among respondents with less trial experience, compared to those with extensive experience (15.7% vs 1.6%; P < .01). Hepatologists and respondents with extensive clinical trial experience tended to be more interested in phase 1 and 2 studies, whereas gastroenterologists and less experienced investigators were more interested in phase 4 studies.
Related collections
Most cited references26
- Record: found
- Abstract: not found
- Article: not found
Translational and clinical science--time for a new vision.
- Record: found
- Abstract: found
- Article: not found
Simply no time? Barriers to GPs' participation in primary health care research.
- Record: found
- Abstract: found
- Article: not found