- Record: found
- Abstract: found
- Article: found
Docosahexaenoic acid attenuates the early inflammatory response following spinal cord injury in mice: in-vivo and in-vitro studies
Read this article at
Abstract
Background
Two families of polyunsaturated fatty acid (PUFA), omega-3 (ω-3) and omega-6 (ω-6), are required for physiological functions. The long chain ω-3 PUFAs, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have significant biological effects. In particular, DHA is a major component of cell membranes in the brain. It is also involved in neurotransmission. Spinal cord injury (SCI) is a highly devastating pathology that can lead to catastrophic dysfunction, with a significant reduction in the quality of life. Previous studies have shown that EPA and DHA can exert neuroprotective effects in SCI in mice and rats. The aim of this study was to analyze the mechanism of action of ω-3 PUFAs, such as DHA, in a mouse model of SCI, with a focus on the early pathophysiological processes.
Methods
In this study, SCI was induced in mice by the application of an aneurysm clip onto the dura mater via a four-level T5 to T8 laminectomy. Thirty minutes after compression, animals received a tail vein injection of DHA at a dose of 250 nmol/kg. All animals were killed at 24 h after SCI, to evaluate various parameters implicated in the spread of the injury.
Results
Our results in this in-vivo study clearly demonstrate that DHA treatment reduces key factors associated with spinal cord trauma. Treatment with DHA significantly reduced: (1) the degree of spinal cord inflammation and tissue injury, (2) pro-inflammatory cytokine expression (TNF-α), (3) nitrotyrosine formation, (4) glial fibrillary acidic protein (GFAP) expression, and (5) apoptosis (Fas-L, Bax, and Bcl-2 expression). Moreover, DHA significantly improved the recovery of limb function.
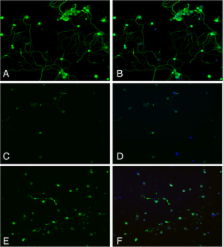
Furthermore, in this study we evaluated the effect of oxidative stress on dorsal root ganglion (DRG) cells using a well-characterized in-vitro model. Treatment with DHA ameliorated the effects of oxidative stress on neurite length and branching.
Related collections
Most cited references26
- Record: found
- Abstract: found
- Article: not found
Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain.
- Record: found
- Abstract: found
- Article: not found
Cellular inflammatory response after spinal cord injury in Sprague-Dawley and Lewis rats.
- Record: found
- Abstract: found
- Article: not found