- Record: found
- Abstract: found
- Article: not found
Development of highly effective LCB1-based lipopeptides targeting the spike receptor-binding motif of SARS-CoV-2

Read this article at
Abstract
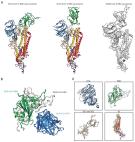
LCB1 is a computationally designed 56-mer miniprotein targeting the spike (S) receptor-binding motif of SARS-CoV- 2 with high potent activity (Science, 2020; Cell host microbe, 2021); however, recent studies have demonstrated that emerging SARS-CoV-2 variants are highly resistant to LCB1's inhibition. In this study, we first identified a truncated peptide termed LCB1v8, which maintained the high antiviral potency. Then, a group of lipopeptides were generated by modifying LCB1v8 with diverse lipids, and of two lipopeptides, the C-terminally stearicacid-conjugtaed LCB1v17 and cholesterol-conjugated LCB1v18, were highly effective in inhibiting both S protein-pseudovirus and authentic SARS-CoV-2 infections. We further showed that LCB1-based inhibitors had similar α-helicity and thermostability in structure and bound to the target-mimic RBD protein with high affinity, and the lipopeptides exhibited greatly enhanced binding with the viral and cellular membranes, improved inhibitory activities against emerging SARS-CoV-2 variants. Moreover, LCB1v18 was validated with high preventive and therapeutic efficacies in K18-hACE2 transgenic mice against lethal SARS-CoV-2 challenge. In conclusion, our studies have provided important information for understanding the structure and activity relationship (SAR) of LCB1 inhibitor and would guide the future development of novel antivirals.
Related collections
Most cited references34
- Record: found
- Abstract: found
- Article: found
Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation
- Record: found
- Abstract: found
- Article: not found
Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein
- Record: found
- Abstract: found
- Article: not found
