- Record: found
- Abstract: found
- Article: found
Immunohistochemistry for detection of avian infectious bronchitis virus strain M41 in the proventriculus and nervous system of experimentally infected chicken embryos
Read this article at
Abstract
Background
Infectious bronchitis virus primarily induces a disease of the respiratory system, different IBV strains may show variable tissue tropisms and also affect the oviduct and the kidneys. Proventriculitis was also associated with some new IBV strains. Aim of this study was to investigate by immunohistochemistry (IHC) the tissue tropism of avian infectious bronchitis virus (IBV) strain M41 in experimentally infected chicken embryos.
Results
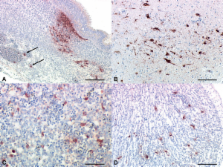
To this end chicken embryos were inoculated in the allantoic sac with 10 3 EID 50 of IBV M41 at 10 days of age. At 48, 72, and 120 h postinoculation (PI), embryos and chorioallantoic membranes (CAM) were sampled, fixed, and paraffin-wax embedded. Allantoic fluid was also collected and titrated in chicken embryo kidney cells (CEK). The sensitivity of IHC in detecting IBV antigens in the CAM of inoculated eggs matched the virus reisolation and detection in CEK. Using IHC, antigens of IBV were detected in nasal epithelium, trachea, lung, spleen, myocardial vasculature, liver, gastrointestinal tract, kidney, skin, sclera of the eye, spinal cord, as well as in brain neurons of the inoculated embryos. These results were consistent with virus isolation and denote the wide tissue tropism of IBV M41 in the chicken embryo. Most importantly, we found infection of vasculature and smooth muscle of the proventriculus which has not seen before with IBV strain M41.
Conclusion
IHC can be an additional useful tool for diagnosis of IBV infection in chickens and allows further studies to foster a deeper understanding of the pathogenesis of infections with IBV strains of different virulence. Moreover, these results underline that embryonic tissues in addition to CAM could be also used as possible source to generate IBV antigens for diagnostic purposes.
Related collections
Most cited references45
- Record: found
- Abstract: found
- Article: not found
Coronaviruses in poultry and other birds.
- Record: found
- Abstract: found
- Article: not found
Recombinant avian infectious bronchitis virus expressing a heterologous spike gene demonstrates that the spike protein is a determinant of cell tropism.
- Record: found
- Abstract: found
- Article: not found