- Record: found
- Abstract: found
- Article: found
Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces
Read this article at
Abstract
Background
Early microbial colonization of the gut reduces the incidence of infectious, inflammatory and autoimmune diseases. Recent population studies reveal that childhood hygiene is a significant risk factor for development of inflammatory bowel disease, thereby reinforcing the hygiene hypothesis and the potential importance of microbial colonization during early life. The extent to which early-life environment impacts on microbial diversity of the adult gut and subsequent immune processes has not been comprehensively investigated thus far. We addressed this important question using the pig as a model to evaluate the impact of early-life environment on microbe/host gut interactions during development.
Results
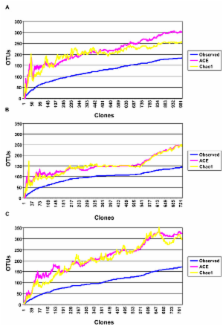
Genetically-related piglets were housed in either indoor or outdoor environments or in experimental isolators. Analysis of over 3,000 16S rRNA sequences revealed major differences in mucosa-adherent microbial diversity in the ileum of adult pigs attributable to differences in early-life environment. Pigs housed in a natural outdoor environment showed a dominance of Firmicutes, in particular Lactobacillus, whereas animals housed in a hygienic indoor environment had reduced Lactobacillus and higher numbers of potentially pathogenic phylotypes. Our analysis revealed a strong negative correlation between the abundance of Firmicutes and pathogenic bacterial populations in the gut. These differences were exaggerated in animals housed in experimental isolators. Affymetrix microarray technology and Real-time Polymerase Chain Reaction revealed significant gut-specific gene responses also related to early-life environment. Significantly, indoor-housed pigs displayed increased expression of Type 1 interferon genes, Major Histocompatibility Complex class I and several chemokines. Gene Ontology and pathway analysis further confirmed these results.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis.
- Record: found
- Abstract: found
- Article: not found
An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system.
- Record: found
- Abstract: not found
- Article: not found