- Record: found
- Abstract: found
- Article: not found
Effectiveness of Adding a Mask Recommendation to Other Public Health Measures to Prevent SARS-CoV-2 Infection in Danish Mask Wearers : A Randomized Controlled Trial

Read this article at
Abstract
Observational evidence suggests that mask wearing mitigates SARS-CoV-2 transmission. It is uncertain if this observed association arises through protection of uninfected wearers (protective effect), via reduced transmission from infected mask wearers (source control), or both. This randomized controlled trial investigates whether recommending surgical mask use when outside the home reduces wearers' risk for SARS-CoV-2 infection in a setting where masks were uncommon and not among recommended public health measures.
Abstract
Observational evidence suggests that mask wearing mitigates SARS-CoV-2 transmission. It is uncertain if this observed association arises through protection of uninfected wearers (protective effect), via reduced transmission from infected mask wearers (source control), or both. This randomized controlled trial investigates whether recommending surgical mask use when outside the home reduces wearers' risk for SARS-CoV-2 infection in a setting where masks were uncommon and not among recommended public health measures.
Abstract
Background:
Observational evidence suggests that mask wearing mitigates transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It is uncertain if this observed association arises through protection of uninfected wearers (protective effect), via reduced transmission from infected mask wearers (source control), or both.
Objective:
To assess whether recommending surgical mask use outside the home reduces wearers' risk for SARS-CoV-2 infection in a setting where masks were uncommon and not among recommended public health measures.
Design:
Randomized controlled trial (DANMASK-19 [Danish Study to Assess Face Masks for the Protection Against COVID-19 Infection]). (ClinicalTrials.gov: NCT04337541)
Participants:
Adults spending more than 3 hours per day outside the home without occupational mask use.
Intervention:
Encouragement to follow social distancing measures for coronavirus disease 2019, plus either no mask recommendation or a recommendation to wear a mask when outside the home among other persons together with a supply of 50 surgical masks and instructions for proper use.
Measurements:
The primary outcome was SARS-CoV-2 infection in the mask wearer at 1 month by antibody testing, polymerase chain reaction (PCR), or hospital diagnosis. The secondary outcome was PCR positivity for other respiratory viruses.
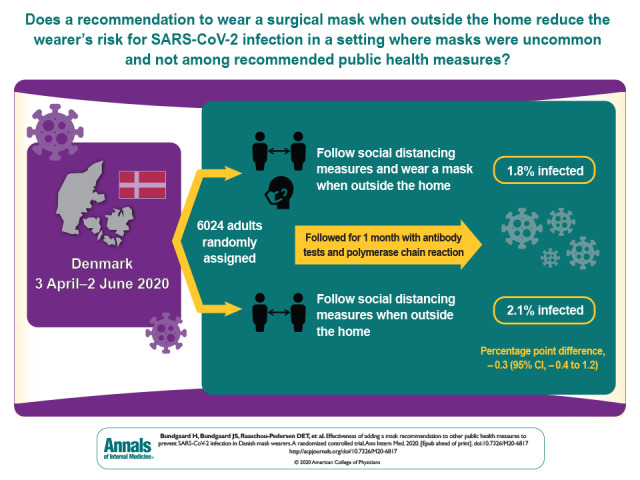
Results:
A total of 3030 participants were randomly assigned to the recommendation to wear masks, and 2994 were assigned to control; 4862 completed the study. Infection with SARS-CoV-2 occurred in 42 participants recommended masks (1.8%) and 53 control participants (2.1%). The between-group difference was −0.3 percentage point (95% CI, −1.2 to 0.4 percentage point; P = 0.38) (odds ratio, 0.82 [CI, 0.54 to 1.23]; P = 0.33). Multiple imputation accounting for loss to follow-up yielded similar results. Although the difference observed was not statistically significant, the 95% CIs are compatible with a 46% reduction to a 23% increase in infection.
Limitation:
Inconclusive results, missing data, variable adherence, patient-reported findings on home tests, no blinding, and no assessment of whether masks could decrease disease transmission from mask wearers to others.
Conclusion:
The recommendation to wear surgical masks to supplement other public health measures did not reduce the SARS-CoV-2 infection rate among wearers by more than 50% in a community with modest infection rates, some degree of social distancing, and uncommon general mask use. The data were compatible with lesser degrees of self-protection.
Related collections
Most cited references35
- Record: found
- Abstract: found
- Article: not found
Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support.
- Record: found
- Abstract: found
- Article: not found
Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1
- Record: found
- Abstract: found
- Article: not found
