- Record: found
- Abstract: found
- Article: found
Potential antipsychotic action of the selective agonist of adenosine A1 receptors, 5′-Cl-5′-deoxy-ENBA, in amphetamine and MK-801 rat models
Read this article at
Abstract
Background
Disturbances of dopaminergic and glutamatergic transmissions have been suggested to be involved in the pathomechanisms underlying psychotic symptoms of schizophrenia. In line with this concept, hyperlocomotion induced by the dopaminomimetic amphetamine and the uncompetitive antagonist of NMDA receptors MK-801 (dizocilpine) in rodents is a generally established model for screening of new potential antipsychotic drugs. Since recent studies have indicated that receptors for adenosine may be targets for antipsychotic therapy, the aim of the present study was to investigate an influence of 5′-Cl-5′-deoxy-ENBA, a potent and selective adenosine A 1 receptor agonist, on hyperlocomotion induced by amphetamine and MK-801.
Methods
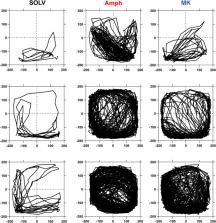
Locomotor activity was measured by Force Plate Actimeters where four force transducers located below the corners of the floor of the cage tracked the animal position on a Cartesian plane at each time point.
Results
Hyperlocomotion induced by either amphetamine (1 mg/kg sc) or MK-801 (0.3 mg/kg ip) was inhibited by 5′-Cl-5′-deoxy-ENBA (0.1 mg/kg ip). The effect of 5′-Cl-5′-deoxy-ENBA on the amphetamine- and MK-801-induced hyperlocomotion was antagonized by the selective antagonist of adenosine A 1 receptor DPCPX at doses of 1 and 2 mg/kg ip, respectively.
Related collections
Most cited references52
- Record: found
- Abstract: found
- Article: not found
NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons.
- Record: found
- Abstract: found
- Article: not found
Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers.
- Record: found
- Abstract: found
- Article: not found