- Record: found
- Abstract: found
- Article: found
The impact of humic acid on metaldehyde adsorption onto powdered activated carbon in aqueous solution†

Abstract
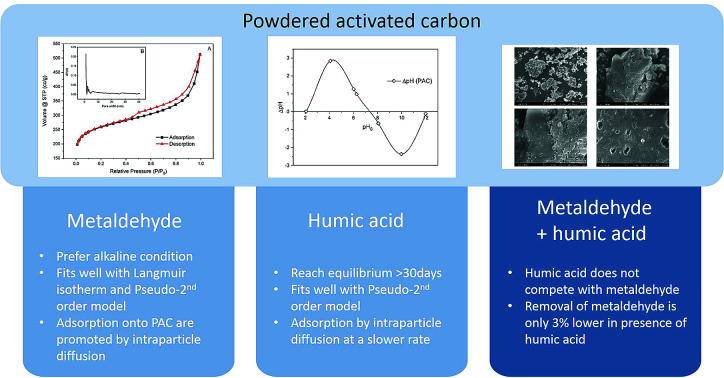
Metaldehyde has been detected in surface water and drinking water in the UK, exceeding the EU and UK standard of 0.1 μg L −1. The presence of natural organic matter (NOM) is considered to affect the removal efficiency of metaldehyde using traditional treatment methods such as adsorption by granular activated carbon. This paper selected humic acid (HA) to represent NOM and investigated the single and binary adsorption systems of metaldehyde and HA by powdered activated carbon (PAC). Metaldehyde was effectively removed by PAC in both systems. Since the percentage removal of metaldehyde was only 3% lower in the binary adsorption system, HA was therefore not considered as a significant compound competing with metaldehyde for adsorption sites on PAC. An adsorption equilibrium study and kinetic study for metaldehyde in a single system suggested that the Langmuir isotherm and the pseudo-second order kinetic model were more suitable in this case than the Freundlich isotherm and the pseudo-first order kinetic model. The two models revealed that the maximum adsorption capacity ( q m) of metaldehyde by PAC was 28.3 mg g −1 and the adsorption rate ( k 2) was 0.16 g mg −1 min −1. The effect of pH of metaldehyde solution was also investigated in a single system. Higher percentage removal of metaldehyde was found under alkaline conditions. In contrast to metaldehyde, HA was not effectively and efficiently removed by PAC in both systems, even with higher PAC dosages and longer contact times. Hence, the microporous and mesoporous PAC was suitable for removing metaldehyde even in the binary system.
Abstract
Powdered activated carbon with abundant micropores and mesopores can effectively remove metaldehyde from aqueous solution in the presence of humic acid.
Related collections
Most cited references9
- Record: found
- Abstract: not found
- Article: not found
Pseudo-first-order kinetic studies of the sorption of acid dyes onto chitosan
- Record: found
- Abstract: found
- Article: found
The application of GAC sandwich slow sand filtration to remove pharmaceutical and personal care products
- Record: found
- Abstract: found
- Article: not found