- Record: found
- Abstract: found
- Article: found
MANTA and MANTA-RAy: Rationale and Design of Trials Evaluating Effects of Filgotinib on Semen Parameters in Patients with Inflammatory Diseases
Read this article at
Abstract
Introduction
The phase 2 MANTA and MANTA-RAy studies were developed in consultation with global regulatory authorities to investigate potential impacts of filgotinib, a Janus kinase 1 preferential inhibitor, on semen parameters in men with active inflammatory diseases. Here we describe the methods and rationale for these studies.
Methods and Rationale
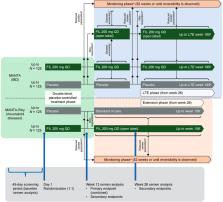
The MANTA and MANTA-RAy studies included men (aged 21–65 years) with active inflammatory bowel disease (IBD) and rheumatic diseases, respectively. Participants had no history of reproductive health issues, and the following semen parameter values (≥ 5th percentile of World Health Organization reference values) at baseline: semen volume ≥ 1.5 mL, total sperm/ejaculate ≥ 39 million, sperm concentration ≥ 15 million/mL, sperm total motility ≥ 40% and normal sperm morphology ≥ 30%. Each trial included a 13-week, randomized, double-blind, placebo-controlled period (filgotinib 200 mg vs placebo, up to N = 125 per arm), for pooled analysis of the week-13 primary endpoint (proportion of participants with ≥ 50% decrease from baseline in sperm concentration). All semen assessments were based on two samples (≤ 14 days apart) to minimize effects of physiological variation; stringent standardization processes were applied across assessment sites. From week 13, MANTA and MANTA-RAy study designs deviated owing to disease-specific considerations. All subjects with a ≥ 50% decrease in sperm parameters continued the study in the monitoring phase until reversibility, or up to a maximum of 52 weeks, with standard of care as treatment. Overall conclusions from MANTA and MANTA-RAy will be based on the totality of the data, including secondary/exploratory measures (e.g. sperm motility/morphology, sex hormones, reversibility of any effects on semen parameters).
Conclusions
Despite the complexities, the MANTA and MANTA-RAy studies form a robust trial programme that is the first large-scale, placebo-controlled evaluation of potential impacts of an advanced IBD and rheumatic disease therapy on semen parameters.
Plain Language Summary
Filgotinib is a treatment for patients with ulcerative colitis and rheumatoid arthritis, and is being studied in other inflammatory diseases. Filgotinib works by blocking Janus kinase 1, an intracellular protein involved in inflammatory signalling processes. We designed the MANTA and MANTA-RAy trials with global health agencies to find out if filgotinib decreases the quality of semen in men with active inflammatory bowel disease (ulcerative colitis or Crohn’s disease) (MANTA) or rheumatic disease (rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis or non-radiographic axial spondylitis) (MANTA-RAy). This paper describes the design of the two trials.
Patients had normal sperm measurements and could not have had previous reproductive health issues. Nearly 250 patients were included in each trial. In both MANTA and MANTA-RAy, half of the patients were treated with 200 mg of filgotinib once a day for 13 weeks, and the other half with placebo. We determined if any patients had a decrease in number of sperm cells per millilitre (sperm concentration) by at least half after 13 weeks of treatment. We then monitored any patients who had such a decrease in sperm concentration for up to 52 weeks (while they received standard of care treatment) or until the decrease was reversed.
The conclusions from the trials will be in a different paper and will be based on all the final data, including changes in sex hormones. This is the first large-scale clinical trial programme to measure the effect of a treatment on sperm in men with inflammatory bowel disease or rheumatic diseases.
Related collections
Most cited references40
- Record: found
- Abstract: found
- Article: found
Chronic inflammation in the etiology of disease across the life span
- Record: found
- Abstract: found
- Article: not found
