- Record: found
- Abstract: found
- Article: found
Advances in Designing and Developing Vaccines, Drugs, and Therapies to Counter Ebola Virus
Read this article at
Abstract
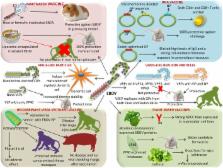
Ebola virus (EBOV), a member of the family Filoviridae, is responsible for causing Ebola virus disease (EVD) (formerly named Ebola hemorrhagic fever). This is a severe, often fatal illness with mortality rates varying from 50 to 90% in humans. Although the virus and associated disease has been recognized since 1976, it was only when the recent outbreak of EBOV in 2014–2016 highlighted the danger and global impact of this virus, necessitating the need for coming up with the effective vaccines and drugs to counter its pandemic threat. Albeit no commercial vaccine is available so far against EBOV, a few vaccine candidates are under evaluation and clinical trials to assess their prophylactic efficacy. These include recombinant viral vector (recombinant vesicular stomatitis virus vector, chimpanzee adenovirus type 3-vector, and modified vaccinia Ankara virus), Ebola virus-like particles, virus-like replicon particles, DNA, and plant-based vaccines. Due to improvement in the field of genomics and proteomics, epitope-targeted vaccines have gained top priority. Correspondingly, several therapies have also been developed, including immunoglobulins against specific viral structures small cell-penetrating antibody fragments that target intracellular EBOV proteins. Small interfering RNAs and oligomer-mediated inhibition have also been verified for EVD treatment. Other treatment options include viral entry inhibitors, transfusion of convalescent blood/serum, neutralizing antibodies, and gene expression inhibitors. Repurposed drugs, which have proven safety profiles, can be adapted after high-throughput screening for efficacy and potency for EVD treatment. Herbal and other natural products are also being explored for EVD treatment. Further studies to better understand the pathogenesis and antigenic structures of the virus can help in developing an effective vaccine and identifying appropriate antiviral targets. This review presents the recent advances in designing and developing vaccines, drugs, and therapies to counter the EBOV threat.
Related collections
Most cited references240
- Record: found
- Abstract: found
- Article: not found
Ebola virus. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment.

- Record: found
- Abstract: found
- Article: found
Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea
- Record: found
- Abstract: found
- Article: not found