- Record: found
- Abstract: found
- Article: found
Estimates of Pandemic Influenza Vaccine Effectiveness in Europe, 2009–2010: Results of Influenza Monitoring Vaccine Effectiveness in Europe (I-MOVE) Multicentre Case-Control Study
Read this article at
Abstract
Results from a European multicentre case-control study reported by Marta Valenciano and colleagues suggest good protection by the pandemic monovalent H1N1 vaccine against pH1N1 and no effect of the 2009–2010 seasonal influenza vaccine on H1N1.
Abstract
Background
A multicentre case-control study based on sentinel practitioner surveillance networks from seven European countries was undertaken to estimate the effectiveness of 2009–2010 pandemic and seasonal influenza vaccines against medically attended influenza-like illness (ILI) laboratory-confirmed as pandemic influenza A (H1N1) (pH1N1).
Methods and Findings
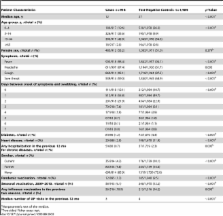
Sentinel practitioners swabbed ILI patients using systematic sampling. We included in the study patients meeting the European ILI case definition with onset of symptoms >14 days after the start of national pandemic vaccination campaigns. We compared pH1N1 cases to influenza laboratory-negative controls. A valid vaccination corresponded to >14 days between receiving a dose of vaccine and symptom onset. We estimated pooled vaccine effectiveness (VE) as 1 minus the odds ratio with the study site as a fixed effect. Using logistic regression, we adjusted VE for potential confounding factors (age group, sex, month of onset, chronic diseases and related hospitalizations, smoking history, seasonal influenza vaccinations, practitioner visits in previous year). We conducted a complete case analysis excluding individuals with missing values and a multiple multivariate imputation to estimate missing values. The multivariate imputation ( n = 2902) adjusted pandemic VE (PIVE) estimates were 71.9% (95% confidence interval [CI] 45.6–85.5) overall; 78.4% (95% CI 54.4–89.8) in patients <65 years; and 72.9% (95% CI 39.8–87.8) in individuals without chronic disease. The complete case ( n = 1,502) adjusted PIVE were 66.0% (95% CI 23.9–84.8), 71.3% (95% CI 29.1–88.4), and 70.2% (95% CI 19.4–89.0), respectively. The adjusted PIVE was 66.0% (95% CI −69.9 to 93.2) if vaccinated 8–14 days before ILI onset. The adjusted 2009–2010 seasonal influenza VE was 9.9% (95% CI −65.2 to 50.9).
Conclusions
Our results suggest good protection of the pandemic monovalent vaccine against medically attended pH1N1 and no effect of the 2009–2010 seasonal influenza vaccine. However, the late availability of the pandemic vaccine and subsequent limited coverage with this vaccine hampered our ability to study vaccine benefits during the outbreak period. Future studies should include estimation of the effectiveness of the new trivalent vaccine in the upcoming 2010–2011 season, when vaccination will occur before the influenza season starts.
Editors' Summary
Background
Following the World Health Organization's declaration of pandemic phase six in June 2009, manufacturers developed vaccines against pandemic influenza A 2009 (pH1N1). On the basis of the scientific opinion of the European Medicines Agency, the European Commission initially granted marketing authorization to three pandemic vaccines for use in European countries. During the autumn of 2009, most European countries included the 2009–2010 seasonal influenza vaccine and the pandemic vaccine in their influenza vaccination programs.
The Influenza Monitoring Vaccine Effectiveness in Europe network (established to monitor seasonal and pandemic influenza vaccine effectiveness) conducted seven case-control and three cohort studies in seven European countries in 2009–2010 to estimate the effectiveness of the pandemic and seasonal vaccines. Data from the seven pilot case-control studies were pooled to provide overall adjusted estimates of vaccine effectiveness.
Why Was This Study Done?
After seasonal and pandemic vaccines are made available to populations, it is necessary to estimate the effectiveness of the vaccines at the population level during every influenza season. Therefore, this study was conducted in European countries to estimate the pandemic influenza vaccine effectiveness and seasonal influenza vaccine effectiveness against people presenting to their doctor with influenza-like illness who were confirmed (by laboratory tests) to be infected with pH1N1.
What Did the Researchers Do and Find?
The researchers conducted a multicenter case-control study on the basis of practitioner surveillance networks from seven countries—France, Hungary, Ireland, Italy, Romania, Portugal, and Spain. Patients consulting a participating practitioner for influenza-like illness had a nasal or throat swab taken within 8 days of symptom onset. Cases were swabbed patients who tested positive for pH1N1. Patients presenting with influenza-like illness whose swab tested negative for any influenza virus were controls.
Individuals were considered vaccinated if they had received a dose of the vaccine more than 14 days before the date of onset of influenza-like illness and unvaccinated if they were not vaccinated at all, or if the vaccine was given less than 15 days before the onset of symptoms. The researchers analyzed pandemic influenza vaccination effectiveness in those vaccinated less than 8 days, those vaccinated between and including 8 and 14 days, and those vaccinated more than 14 days before onset of symptoms compared to those who had never been vaccinated.
The researchers used modeling (taking account of all potential confounding factors) to estimate adjusted vaccine effectiveness and stratified the adjusted pandemic influenza vaccine effectiveness and the adjusted seasonal influenza vaccine effectiveness in three age groups (<15, 15–64, and ≥65 years of age).
The adjusted results suggest that the 2009–2010 seasonal influenza vaccine did not protect against pH1N1 illness. However, one dose of the pandemic vaccines used in the participating countries conferred good protection (65.5%–100% according to various stratifications performed) against pH1N1 in people who attended their practitioner with influenza-like illness, especially in people aged <65 years and in those without any chronic disease. Furthermore, good pandemic influenza vaccine effectiveness was observed as early as 8 days after vaccination.
What Do These Findings Mean?
The results of this study provide early estimates of the pandemic influenza vaccine effectiveness suggesting that the monovalent pandemic vaccines have been effective. The findings also give an indication of the vaccine effectiveness for the Influenza A (H1N1) 2009 strain included in the 2010–2011 seasonal vaccines, although specific vaccine effectiveness studies will have to be conducted to verify if similar good effectiveness are observed with 2010–2011 trivalent vaccines. However, the results of this study should be interpreted with caution because of limitations in the pandemic context (late timing of the studies, low incidence, low vaccine coverage leading to imprecise estimates) and potential biases due the study design, confounding factors, and missing values. The researchers recommend that in future season studies, the sample size per country should be enlarged in order to allow for precise pooled and stratified analyses.
Additional Information
Please access these websites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed.1000388.
-
The World Health Organization has information on H1N1 vaccination
-
The US Centers for Disease Control and Prevention provides a fact sheet on the 2009 H1N1 influenza virus
-
The US Department of Health and Human services has a comprehensive website on flu
-
The European Centre for Disease Prevention and Control provides information on 2009 H1N1 pandemic
-
The European Centre for Disease Prevention and Control presents a summary of the 2009 H1N1 pandemic in Europe and elsewhere
Related collections
Most cited references19
- Record: found
- Abstract: found
- Article: not found
Effectiveness of inactivated influenza vaccines varied substantially with antigenic match from the 2004-2005 season to the 2006-2007 season.
- Record: found
- Abstract: found
- Article: not found
Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine.
- Record: found
- Abstract: found
- Article: not found