- Record: found
- Abstract: found
- Article: found
British Society of Gastroenterology interim framework for addressing the COVID-19-related backlog in inflammatory bowel disease colorectal cancer surveillance
article-commentary

Antonia MD Churchhouse
1 ,
Victoria EL Moffat
1 ,
Christian P Selinger
2 ,
Christopher A Lamb
3
,
4 ,
Michelle J Thornton
5 ,
Ian Penman
6 ,
Shahida Din
1
,
,
the BSG, Scottish IBD Surveillance Group
5 September 2022
Read this article at
There is no author summary for this article yet. Authors can add summaries to their articles on ScienceOpen to make them more accessible to a non-specialist audience.
Abstract
The COVID-19 pandemic has had profound implications across the breadth of national
healthcare services. Inflammatory bowel disease (IBD) continues to carry an increased
risk of colon cancer and national protocols for endoscopic surveillance are in place.
Elective procedures such as IBD surveillance were stopped during the COVID-19 pandemic
and have been slow to be re-started. We are acutely aware of the pressures on endoscopy
services at the present time which is unlikely to improve at the pace needed for services
to fully recover. At such times, we need to target this scarce resource to those who
need it most, aligned to the principles of ethical healthcare which state that when
resources are limited, they should be used to provide the most benefit for as great
a number of people as possible. With this in mind, we propose an optional interim
framework to aid risk stratification of patients on the IBD surveillance waiting lists
where delays to timely surveillance occur. These measures could help address the backlog
until a time when clinical services are able to fully recover. Finally, we propose
the patient factors to consider when withdrawal of surveillance may be contemplated.
Key points
There is significant variation in the degree of endoscopy surveillance backlog across
the UK.
For centres without a significant backlog, no major change to clinical practice is
likely to be needed.
For centres with a significant backlog, we recommend employing a framework to aid
risk stratification of patients on the IBD surveillance waiting list.
Those patients at highest risk should continue to be offered urgent surveillance colonoscopy
at the earliest opportunity.
We propose an interim framework where patients at medium to low risk can be further
triaged using, quantitative Faecal Immunochemical Test for haemoglobin (qFIT) and
faecal calprotectin, stool tests used routinely in clinical practice.
Patients with a high qFIT (≥10 µg/g) and low calprotectin (<250 µg/g) should be treated
as high risk for cancer and may therefore reflect those in greatest need of surveillance.
We also consider how the demand for surveillance procedures could be addressed by
postponing and/or withdrawing patients from surveillance programmes if the overall
risk of future dysplasia/cancer is low and unlikely to occur in the patient’s expected
lifespan.
IBD dysplasia surveillance
IBD confers an increased risk of inflammation-associated colorectal cancer (CRC),
herein termed IBD-CRC. The prevalence of IBD in the Western world, and therefore the
population in need of surveillance, is increasing due to static incidence and low
mortality.1 However, across the UK only 63% of all endoscopy surveillance procedure
targets were met prior to the pandemic in 2019.2 Despite surveillance, it is estimated
that 28–41% of IBD-CRCs present as interval cancers3 (post-colonoscopy cancers) between
surveillance procedures. In the current post-pandemic environment, urgent strategies
are required to identify those patients most likely to benefit from surveillance,
while mitigating potential harm to those patients on wating lists.
The impact of the COVID-19 pandemic on IBD surveillance
In March 2020, the British Society of Gastroenterology (BSG) and the Joint Advisory
Group advised a 6-week pause in non-emergency endoscopy in the wake of the COVID-19
pandemic.4 During this time, overall endoscopy activity reduced to 5% of pre-pandemic
levels and has slowly recovered to near pre-pandemic levels,5 with a backlog of procedures
awaiting to be scheduled. Data from the pandemic are emerging, with evidence of a
significant reduction in 2-week wait referrals and CRC diagnoses. In Scotland, there
was a 19% reduction in CRC diagnoses in 2020 compared with 20196; reflecting a reduction
in clinical activity and not a true decrease in cancer rate. The Gastroenterology
‘Getting it Right First Time’ (GIRFT) national report has highlighted that Endoscopy
units are continuing to perform at reduced capacity due to ongoing COVID-19 pandemic
constraints and English Trusts have reported significant concerns in managing the
substantial backlog in surveillance procedures.7 As of 31 December 2021, there were
34 224 patients waiting for an endoscopy in Scotland (including colonoscopy), a 53.1%
increase compared with the 12-month average prior to the onset of the pandemic.8 In
England in March 2022 there were 159 672 patients on the endoscopy waiting list, with
66 512 waiting for a colonoscopy.9
While the backlog of procedures overall is significant, surveillance procedures have
been slower still to recover.10 Additional BSG advice was issued in April 2021 regarding
surveillance colonoscopy (including for IBD) in the wake of these challenges, suggesting
surveillance procedures should be carried out within 6–12 months of their due date.11
It is concerning that most patients will have already surpassed this timepoint, and
thus additional measures are needed to help prioritise those awaiting surveillance
procedures.
Alternative strategies, such as the qFIT and virtual colonoscopy (either via CT or
capsule), have not been used in IBD surveillance. qFIT, which measures the concentration
of degraded haemoglobin and is raised in ulcerative colitis (UC) patients with active
inflammation,12 13 has not been validated as a marker of IBD-related dysplasia. Virtual
colonoscopy via CT or capsule imaging may not be sufficiently sensitive to detect
flat dysplastic lesions associated IBD-CRC. While there is a lack of data on the rate
of post-colonoscopy colorectal cancer (PCCRC) in the wake of the COVID-19 pandemic,
the high rate of pre-pandemic PCCRC, together with the backlog of procedures, raises
concerns that this rate could increase further as the time interval between surveillance
extends. Such concerns have also been reported in the media.14
Possible strategies to tackle current challenges in IBD surveillance colonoscopy for
centres with a significant backlog in procedures
For some centres without a backlog in surveillance procedures, no change may be required.
For centres with a significant backlog and under unprecedented pressure, we first
propose that colonoscopy waiting lists be reviewed and stratified, such that patients
with IBD are identified (through International Classification of Diseases 10 codes,
faecal calprotectin, mesalazine prescription, for example1 or using the IBD Registry,15
if available), and triaged according to the existing surveillance framework.16 While
we have not set a specific lower age limit for this framework, we do not anticipate
it applying to most paediatric IBD centres.
Identifying these patients on undifferentiated waiting lists can be challenging. We
recognise that this level of waiting list validation will require additional resources.
Acknowledging the significant numbers of patients who are likely to be overdue surveillance,
we propose that clinical risk stratification should be used to prioritise those at
highest risk of dysplasia. Those due annual colonoscopy, including those with extensive
moderately to severely active colitis or primary sclerosing cholangitis,17 previous
IBD-related dysplasia or cancer or colonic stricture or with a first degree relative
with CRC diagnosed at <50 years are highest risk and should be scheduled immediately.16
Those not due annual colonoscopy are deemed moderate or low risk and may be further
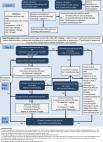
triaged using the proposals described in figure 1.
Figure 1
Proposed algorithm for the management of patients currently on moderate to low risk
IBD surveillance waiting list where significant delays to surveillance are experienced.
A faecal calprotectin threshold of >250 µg/g to indicate disease activity is based
on consensus and published evidence.31–33 A three-point colonoscopy indicates a 45-minute
procedure. 1st DR, first degree relative; CRC, colorectal cancer;FH, family history;
IBD, inflammatory bowel disease; PSC, primary sclerosing cholangitis; qFIT, quantitative
Faecal Immunochemical Test for haemoglobin
The Gastroenterology GIRFT report has recommended the use of stool biomarkers to aid
in the prioritisation of colonoscopy procedures on waiting lists. Faecal calprotectin
is widely used in patients with IBD. qFIT has been shown to preform equally18 or superiorly19
than faecal calprotectin in identifying mucosal healing in UC. Using qFIT in this
way is an untested strategyalthough similar COVID-19 adapted colorectal cancer pathways
have been used for symptomatic patients using qFIT.20 Extrapolated from the work in
symptomatic colorectal patients, a qFIT cut-off of 10 µg/g could be used to aid prioritisation.21
Patients who have a qFIT of ≥10 µg/g could be prioritised for more urgent procedures
with further prioritisation if the level is ≥400 µg/g.22 qFIT in combination with
faecal calprotectin may be a promising risk stratification tool and the combination
of qFIT and calprotectin in one study has been shown to have a higher specificity
for histological healing than when using qFIT alone.18 Therefore, stratifying patients
by both qFIT and faecal calprotectin could provide an indication as to those in most
urgent need of surveillance colonoscopy. In figure 1, we present a model of how this
could be undertaken depending on the potential outcomes for both tests in tandem.
For example, a patient who has a faecal calprotectin level suggestive of remission
(<250 µg/g) and a qFIT ≥10 µg/g may have serious non-inflammatory pathology and could
be prioritised in the waiting list. Alternatively, a patient with a negative qFIT
<10 µg/g and low calprotectin are at a lower risk and could remain on the current
waiting list without prioritisation. In the latter scenario, the negative predictive
value of a qFIT is exploited while accepting the predicted poor sensitivity and specificity
of a positive test in those with active IBD. We have proposed a calprotectin cut-off
of 250 µg/g as this is commonly used in clinical practice, but an alternative of <150 µg/g
has been proposed as a more stringent end point for mucosal healing.23
Patients with a high faecal calprotectin are also likely to have a high qFIT suggesting
active IBD and therefore optimising IBD treatment before conducting a surveillance
procedure would be a reasonable approach, given the challenges of surveying an inflamed
colon and the association with PCCRC rate.24 Importantly, capturing the patients who
are stratified using these proposals in a database with prospective data collection
linked with colonoscopy outcomes will inform the risk stratification and future guidelines.
When can cessation of surveillance be safely considered?
In addition to targeting endoscopic surveillance to those most at risk and using a
combination of biomarkers and risk factors to identify these patients, we should also
be reviewing the appropriateness of ongoing surveillance. Although prompted by the
situation we currently face, this aligns with the principle of sustainable healthcare,
in reducing unnecessary procedures. We recognise the effects on healthcare usage and
expenditure but also highlight the potential reduction in need for invasive procedures
often disliked by patients and carrying small procedure-related risks.25 The current
cessation advice is that surveillance should cease when the ‘risks of the procedure
and its associated implications outweigh the benefits’.26 In practice, identifying
individuals who will benefit from cessation of surveillance is less likely to have
an age-specific cut-off, and more likely to involve both patient engagement and clinical
features.27 The aim of surveillance for IBD-CRC is to prevent premature death from
CRC and we therefore need to consider the likelihood of IBD-CRC occurring during the
patient’s expected remaining life span. Two retrospective studies have suggested that
a lack of histological inflammation in two consecutive procedures predict a low risk
of future CRC.28 29 This approach could be considered for patients who are currently
in the 5-year follow-up category based on the BSG guidance, freeing up capacity for
higher-risk patients.
Cessation of surveillance considerations should ideally be formally addressed by a
future BSGGrading of Recommendations, Assessment, Development and Evaluations (GRADE)
compliant guideline process. Until that time, in the post-pandemic setting, cessation
of IBD surveillance could be considered at the age of 75 years after a good quality
normal procedure, as the expected natural lifespan30 would be fewer years than the
time taken to develop dysplasia and CRC. Second, stopping surveillance after two good
quality consecutive normal procedures, (and at the time of quiescent disease), where
no dysplasia or cancer is detected, could also be considered. Patients in whom surveillance
is suspended should remain under regular review and be offered regular qFIT and faecal
calprotectin tests to mitigate a future delayed diagnosis of CRC. Importantly, surveillance
could be restarted if a patient develops a future flare. Additionally, patients in
whom surveillance is suspended using this interim framework should be captured in
a database and their ongoing surveillance need reviewed once GRADE-compliant consensus
guidance on IBD surveillance cessation is available. This temporary post-pandemic
approach would help to ensure that patients who are in greatest need of surveillance
are appointed first.
Patient engagement
Feedback on the proposed changes were captured by a focus group invitation through
social media publicised by the UK patient charity Crohn’s & Colitis UK. Eight participants
(6F:2M) living with IBD (4 Crohn's Disease:4 UC) for more than 5 years in the age
groups 25 to over 65 years, responded to the invitation of whom six had experience
of regular surveillance procedures.
There was a unanimous positive response to developing a strategy addressing the COVID-19-related
backlog in surveillance procedures. A non-invasive approach to guide surveillance
colonoscopy timing was welcomed, including prioritising those at higher risk. Many
patients are familiar with stool tests and therefore this seemed a reasonable approach.
A single stool sample is often a ‘snapshot’ of disease at time and participants were
reassured that disease flares will be pro-actively managed as in figure 1. The participants
reported that colonoscopy can be an uncomfortable procedure and therefore an approach
which could tailor procedures safely without compromising care would be greatly received
(further detailed responses from seven of the eight participants can be found in online
supplemental appendix 1).
10.1136/gutjnl-2022-328309.supp1
Supplementary data
Conclusion
There are significant ongoing barriers to cancer surveillance in patients with IBD
as a consequence of the COVID-19 pandemic. There is no perfect solution to the growing
pressures endoscopy units face. Leaving patients with IBD on long overdue surveillance
waiting lists in a permanent ‘holding’ pattern, or conversely, putting people at very
low risk through invasive procedures for little benefit, is equally not acceptable.
There is an urgent requirement to survey those patients at highest risk. We propose
an interim mitigation strategy, pending formal GRADE-compliant consensus guidance,
for those not at highest risk using a combination of qFIT and faecal calprotectin
prioritising those in need of endoscopic assessment now. Clinical units adopting this
approach are encouraged to prospectively collect data, as rigorous data collection,
analysis and safety netting will be crucial in determining the impact of the interim
proposals. Regular review of waiting lists, effective triage and resources assigned
appropriately to facilitate this are also important steps to ensure an effective safe
service in the post-pandemic phase.
Related collections
Most cited references24
- Record: found
- Abstract: found
- Article: found
British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults
Christopher Lamb, Nicholas Kennedy, Tim Raine … (2019)
- Record: found
- Abstract: found
- Article: not found
Impact of the COVID-19 pandemic on UK endoscopic activity and cancer detection: a National Endoscopy Database Analysis
Matthew Rutter, Matthew Brookes, Thomas J Lee … (2020)

- Record: found
- Abstract: found
- Article: found
IBD prevalence in Lothian, Scotland, derived by capture–recapture methodology
Gareth‐Rhys Jones, Mathew Lyons, Nikolas Plevris … (2019)