- Record: found
- Abstract: found
- Article: found
Hydrocarbon seepage in the deep seabed links subsurface and seafloor biospheres
Read this article at
Significance
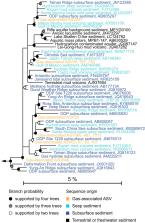
The marine subsurface is one of the largest habitats on Earth composed exclusively of microorganisms and harboring on the order of 10 29 microbial cells. It is unclear if deep subsurface life impacts overlying seafloor diversity and biogeochemical cycling in the deep ocean. We analyzed the microbial communities of 172 seafloor surface sediment samples, including gas and oil seeps as well as sediments not subject to upward fluid flow. A strong correlation between typical subsurface clades and active geofluid seepage suggests that subsurface life is injected into the deep ocean floor at hydrocarbon seeps, a globally widespread hydrogeological phenomenon. This supply of subsurface-derived microbial populations, biomass, and metabolic potential thus increases biodiversity and impacts carbon cycling in the deep ocean.
Abstract
Marine cold seeps transmit fluids between the subseafloor and seafloor biospheres through upward migration of hydrocarbons that originate in deep sediment layers. It remains unclear how geofluids influence the composition of the seabed microbiome and if they transport deep subsurface life up to the surface. Here we analyzed 172 marine surficial sediments from the deep-water Eastern Gulf of Mexico to assess whether hydrocarbon fluid migration is a mechanism for upward microbial dispersal. While 132 of these sediments contained migrated liquid hydrocarbons, evidence of continuous advective transport of thermogenic alkane gases was observed in 11 sediments. Gas seeps harbored distinct microbial communities featuring bacteria and archaea that are well-known inhabitants of deep biosphere sediments. Specifically, 25 distinct sequence variants within the uncultivated bacterial phyla Atribacteria and Aminicenantes and the archaeal order Thermoprofundales occurred in significantly greater relative sequence abundance along with well-known seep-colonizing members of the bacterial genus Sulfurovum, in the gas-positive sediments. Metabolic predictions guided by metagenome-assembled genomes suggested these organisms are anaerobic heterotrophs capable of nonrespiratory breakdown of organic matter, likely enabling them to inhabit energy-limited deep subseafloor ecosystems. These results point to petroleum geofluids as a vector for the advection-assisted upward dispersal of deep biosphere microbes from subsurface to surface environments, shaping the microbiome of cold seep sediments and providing a general mechanism for the maintenance of microbial diversity in the deep sea.
Related collections
Most cited references38
- Record: found
- Abstract: found
- Article: not found
Microbial ecology of the dark ocean above, at, and below the seafloor.
- Record: found
- Abstract: found
- Article: not found
Biogeographical distribution and diversity of microbes in methane hydrate-bearing deep marine sediments on the Pacific Ocean Margin.
- Record: found
- Abstract: found
- Article: not found