- Record: found
- Abstract: found
- Article: found
COVID-19 vaccine coverage, safety, and perceptions among patients with diabetes mellitus in China: a cross-sectional study
Read this article at
Abstract
Aims
Diabetes mellitus (DM), one of the most common chronic diseases in China, is a risk factor for SARS-COV-2 infection and poor prognosis of COVID-19. The COVID-19 vaccine is one of the key measures to control the pandemic. However, the actual coverage of COVID-19 vaccination and associated factors remain unclear among DM patients in China. We conducted this study to investigate the COVID-19 vaccine coverage, safety, and perceptions among patients with DM in China.
Methods
A cross-sectional study of a sample of 2200 DM patients from 180 tertiary hospitals in China was performed using a questionnaire developed through the Wen Juan Xing survey platform to collect information regarding their coverage, safety, and perceptions of COVID-19 vaccination. A multinomial logistic regression analysis model was performed to determine any independent relationships with COVID-19 vaccination behavior among DM patients.
Results
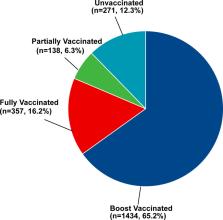
In total, 1929 (87.7%) DM patients have received at least one dose COVID-19 vaccine, and 271 (12.3%) DM patients were unvaccinated. In addition, 65.2% (n = 1434) were booster vaccinated against COVID-19, while 16.2% (n = 357) were only fully vaccinated and 6.3% (n = 138) were only partially vaccinated. The prevalence of adverse effects after the first dose of vaccine, the second dose of vaccine, and the third dose of vaccine were 6.0%, 6.0%, and 4.3% respectively. Multinomial logistic regression analysis showed that DM patients complicated with immune and inflammatory diseases (partially vaccinated: OR = 0.12; fully vaccinated: OR = 0.11; booster vaccinated: OR = 0.28), diabetic nephropathy (partially vaccinated: OR = 0.23; fully vaccinated: OR = 0.50; booster vaccinated: OR = 0.30), and perceptions on the safety of COVID-19 vaccine (partially vaccinated: OR = 0.44; fully vaccinated: OR = 0.48; booster vaccinated: OR = 0.45) were all associated with the three of vaccination status.
Conclusion
This study showed that higher proportion of COVID-19 vaccine coverage among patients with DM in China. The concern about the safety of the COVID-19 vaccine affected the vaccine behavior in patients with DM. The COVID-19 vaccine was relatively safe for DM patients due to all side effects were self-limiting.
Related collections
Most cited references58
- Record: found
- Abstract: found
- Article: not found